Complex II ambiguities: Difference between revisions
No edit summary |
No edit summary |
||
| Line 6: | Line 6: | ||
== FADH<sub>2</sub> and S-pathway == | == FADH<sub>2</sub> and S-pathway == | ||
:::: [[File:Arnold, Finley 2022 CORRECTION.png|800px|link=Arnold 2022 J Biol Chem]] | :::: [[File:Arnold, Finley 2022 CORRECTION.png|800px|link=Arnold 2022 J Biol Chem]] | ||
<br> | |||
:::: [[File:Turton 2022 Int J Mol Sci CORRECTION.png|800px|link=Turton 2022 Int J Mol Sci]] | |||
<br> | |||
:::: [[File:Ahmad 2022 StatPearls CORRECTION.png|800px|link=Ahmad 2022 StatPearls Publishing]] | |||
<br> | |||
:::: [[File:Martinez-Reyes, Chandel 2020 CORRECTION.png|800px|link=Martinez-Reyes 2020 Nat Commun]] | :::: [[File:Martinez-Reyes, Chandel 2020 CORRECTION.png|800px|link=Martinez-Reyes 2020 Nat Commun]] | ||
<br> | |||
:::: [[File:Yepez 2018 PLOS One Fig1B.jpg|400px|left|link=Yepez 2018 PLOS One]] | |||
<br> | |||
:::: [[File:DeBerardinis, Chandel 2016 CORRECTION.png|800px|link=DeBerardinis 2016 Sci Adv]] | :::: [[File:DeBerardinis, Chandel 2016 CORRECTION.png|800px|link=DeBerardinis 2016 Sci Adv]] | ||
<br> | |||
:::: [[File:Sanchez et al 2001 CORRECTION.png|800px|link=Sanchez 2001 Br J Pharmacol]] | :::: [[File:Sanchez et al 2001 CORRECTION.png|800px|link=Sanchez 2001 Br J Pharmacol]] | ||
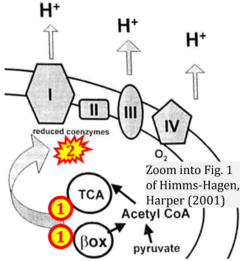
:::: [[File:Himms-Hagen, Harper 2001 CORRECTION.png|250px | <br> | ||
:::* FADH<sub>2</sub> appears in several publications as the substrate of CII in the electron transfer system. This commonly found error requires correction. For clarification, see | |||
:::: [[File:Himms-Hagen, Harper 2001 CORRECTION.png|250px|link=Himms-Hagen 2001 Exp Biol Med (Maywood)]] [[File:OpenStax Biology.png|400px]] | |||
<br> | |||
:::* FADH<sub>2</sub> appears in several publications as the substrate of CII in the electron transfer system. This commonly found error requires correction. For clarification, see [[Gnaiger_2020_BEC_MitoPathways |Gnaiger (2020) page 48]]. | |||
:::* '''Links to FADH<sub>2</sub>→CII misconceptions''' | |||
::::* [[Arnold 2022 J Biol Chem]] | ::::* [[Arnold 2022 J Biol Chem]] | ||
::::* [[Turton 2022 Int J Mol Sci]] | |||
::::* [[Ahmad 2022 StatPearls Publishing]] | |||
::::* [[Martinez-Reyes 2020 Nat Commun]] | ::::* [[Martinez-Reyes 2020 Nat Commun]] | ||
::::* [[Yepez 2018 PLOS One]] | ::::* [[Yepez 2018 PLOS One]] | ||
::::* [[Zhang 2018 Mil Med Res]] | |||
::::* [[DeBerardinis 2016 Sci Adv]] | ::::* [[DeBerardinis 2016 Sci Adv]] | ||
::::* [[Himms-Hagen 2001 Exp Biol Med (Maywood)]] | ::::* [[Himms-Hagen 2001 Exp Biol Med (Maywood)]] | ||
::::* [[Sanchez 2001 Br J Pharmacol]] | ::::* [[Sanchez 2001 Br J Pharmacol]] | ||
::::* [https://openstax.org/books/biology/pages/7-4-oxidative-phosphorylation Oxidative phosphorylation] by OpenStax Biology (CC BY 3.0) got it wrong in figures and text, and the error is propagated further, with copies in | |||
::::::* [https://www.khanacademy.org/science/ap-biology/cellular-energetics/cellular-respiration-ap/a/oxidative-phosphorylation-etc Khan Academy] | |||
::::::* [https://bio.libretexts.org/Bookshelves/Introductory_and_General_Biology/Book%3A_General_Biology_(Boundless)/07%3A_Cellular_Respiration/7.11%3A_Oxidative_Phosphorylation_-_Electron_Transport_Chain LibreTexts Biology] | |||
::::::* [https://learn.saylor.org/mod/page/view.php?id=32815 saylor.org Academy] | |||
::::::* [https://courses.lumenlearning.com/wm-biology1/chapter/reading-electron-transport-chain/ lumen Biology for Majors I] | |||
::::::* [https://jackwestin.com/resources/mcat-content/oxidative-phosphorylation/electron-transfer-in-mitochondria Jack Westin MCAT Courses] | |||
::::* [https://conductscience.com/electron-transport-chain/ Conduct Science]:"In Complex II, the enzyme succinate dehydrogenase in the inner mitochondrial membrane reduce FADH<sub>2</sub> to FAD<sup>+</sup>. Simultaneously, succinate, an intermediate in the Krebs cycle, is oxidized to fumarate." - Comments: FAD does not have a postive charge. FADH<sub>2</sub> is the reduced form, it is not reduced. ''And again:'' In CII, FAD is reduced to FADH<sub>2</sub>. | |||
== CII and fatty acid oxidation == | == CII and fatty acid oxidation == | ||
:::: "Since mitochondrial Complex II also participates in the oxidation of fatty acids (6), .." (quote from [ Lemmi et al 1990]). | ::: CII is not involved in fatty acid oxidation, which requires electron transferring flavoprotein CETF and CI. But there are confusing accounts of a rote of CII in fatty acid oxidation, which require correction: | ||
::::* "Since mitochondrial Complex II also participates in the oxidation of fatty acids (6), .." (quote from [ Lemmi et al 1990]). | |||
::::::* Ref 6: Tzagoloff A (1982) Mitochondria. Plenum, New York. | ::::::* Ref 6: Tzagoloff A (1982) Mitochondria. Plenum, New York. | ||
::::* [https://www.chem.purdue.edu/courses/chm333/Spring%202013/Lectures/Spring%202013%20Lecture%2037%20-%2038.pdf CHM333 LECTURES 37 & 38: 4/27 – 29/13 SPRING 2013 Professor Christine Hrycyna] - Acyl-CoA dehydrogenase is listed under 'Electron transfer in Complex II'. | |||
{{MitoPedia concepts | {{MitoPedia concepts | ||
|mitopedia concept=MiP concept | |mitopedia concept=MiP concept | ||
| Line 26: | Line 62: | ||
{{MitoPedia topics | {{MitoPedia topics | ||
|mitopedia topic=Enzyme, Substrate and metabolite | |mitopedia topic=Enzyme, Substrate and metabolite | ||
}} | |||
{{Labeling | |||
|additional=MitoPedia:FAT4BRAIN | |||
}} | }} | ||
Revision as of 02:55, 27 February 2023
Description
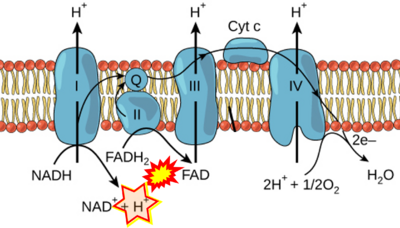
The Succinate pathway is represented in the literature in some cases with a surprising confusion which warrants an analysis of Complex II ambiguities. The CII ambiguity has its roots in the narrative that reduced coenzymes (NADH and FADH2) feed electrons from the Krebs cycle into the membrane-bound electron transfer system. In corresponding ambiguous graphical representations, CII in the canonical ('forward') Krebs cycle is shown to reduce FAD to FADH2 (correct), yet CII in the membrane-bound electron transfer system is paradoxically represented as the site of oxidation of FADH2 to FAD. For clarification: "The substrate of CII is succinate, which is oxidized forming fumarate while reducing flavin adenine dinucleotide FAD to FADH2, with further electron transfer to the quinone pool. Whereas reduced NADH is a substrate of Complex I linked to dehydrogenases of the TCA cycle and mt-matrix upstream of CI, reduced FADH2 is a product of Complex II with downstream electron flow from CII to Q" (quote from Gnaiger 2020).
Abbreviation: CII ambiguities
Reference: Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Bioenerg Commun 2020.2. https://doi.org/10.26124/bec:2020-0002
FADH2 and S-pathway
- FADH2 appears in several publications as the substrate of CII in the electron transfer system. This commonly found error requires correction. For clarification, see Gnaiger (2020) page 48.
- Links to FADH2→CII misconceptions
- Arnold 2022 J Biol Chem
- Turton 2022 Int J Mol Sci
- Ahmad 2022 StatPearls Publishing
- Martinez-Reyes 2020 Nat Commun
- Yepez 2018 PLOS One
- Zhang 2018 Mil Med Res
- DeBerardinis 2016 Sci Adv
- Himms-Hagen 2001 Exp Biol Med (Maywood)
- Sanchez 2001 Br J Pharmacol
- Oxidative phosphorylation by OpenStax Biology (CC BY 3.0) got it wrong in figures and text, and the error is propagated further, with copies in
- Conduct Science:"In Complex II, the enzyme succinate dehydrogenase in the inner mitochondrial membrane reduce FADH2 to FAD+. Simultaneously, succinate, an intermediate in the Krebs cycle, is oxidized to fumarate." - Comments: FAD does not have a postive charge. FADH2 is the reduced form, it is not reduced. And again: In CII, FAD is reduced to FADH2.
CII and fatty acid oxidation
- CII is not involved in fatty acid oxidation, which requires electron transferring flavoprotein CETF and CI. But there are confusing accounts of a rote of CII in fatty acid oxidation, which require correction:
- "Since mitochondrial Complex II also participates in the oxidation of fatty acids (6), .." (quote from [ Lemmi et al 1990]).
- Ref 6: Tzagoloff A (1982) Mitochondria. Plenum, New York.
- CII is not involved in fatty acid oxidation, which requires electron transferring flavoprotein CETF and CI. But there are confusing accounts of a rote of CII in fatty acid oxidation, which require correction:
- CHM333 LECTURES 37 & 38: 4/27 – 29/13 SPRING 2013 Professor Christine Hrycyna - Acyl-CoA dehydrogenase is listed under 'Electron transfer in Complex II'.
MitoPedia concepts:
MiP concept
MitoPedia topics:
Enzyme,
Substrate and metabolite
Labels:
MitoPedia:FAT4BRAIN