Bernardo 2013 Biol Chem: Difference between revisions
(Created page with "{{Publication |title=Bernardo A, De Simone R, De Nuccio C, Visentin S, Minghetti L (2013) The nuclear receptor peroxisome proliferator-activated receptor-γ promotes oligodend...") |
No edit summary |
||
| Line 8: | Line 8: | ||
|editor=Gnaiger E | |editor=Gnaiger E | ||
}} | }} | ||
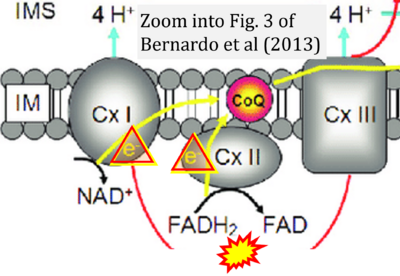
[[File:Bernardo 2013 Biol Chem CORRECTION.png|right|400px]] | |||
{{Template:Correction FADH2 and S-pathway}} | |||
{{Labeling | {{Labeling | ||
|enzymes=Complex II;succinate dehydrogenase | |enzymes=Complex II;succinate dehydrogenase | ||
}} | }} | ||
Revision as of 22:05, 3 August 2023
| Bernardo A, De Simone R, De Nuccio C, Visentin S, Minghetti L (2013) The nuclear receptor peroxisome proliferator-activated receptor-γ promotes oligodendrocyte differentiation through mechanisms involving mitochondria and oscillatory Ca2+ waves. Biol Chem 394:1607-14. doi: 10.1515/hsz-2013-0152 |
Bernardo A, De Simone R, De Nuccio C, Visentin S, Minghetti L (2013) Biol Chem
Abstract: Peroxisome proliferator-activated receptor-γ (PPAR-γ) is one of the most studied nuclear receptor since its identification as a target to treat metabolic and neurological diseases. In addition to exerting anti-inflammatory and neuroprotective effects, PPAR-γ agonists, such as the insulin-sensitizing drug pioglitazone, promote the differentiation of oligodendrocytes (OLs), the myelin-forming cells of the central nervous system (CNS). In addition, PPAR-γ agonists increase OL mitochondrial respiratory chain activity and OL's ability to respond to environmental signals with oscillatory Ca2+ waves. Both OL maturation and oscillatory Ca2+ waves are prevented by the mitochondrial inhibitor rotenone and restored by PPAR-γ agonists, suggesting that PPAR-γ promotes myelination through mechanisms involving mitochondria.
• Bioblast editor: Gnaiger E
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Labels:
Enzyme: Complex II;succinate dehydrogenase