DTPA: Difference between revisions
From Bioblast
(replaced FW by M and added units g*mol-1) |
No edit summary |
||
| (7 intermediate revisions by 4 users not shown) | |||
| Line 3: | Line 3: | ||
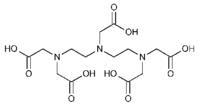
|description='''DTPA''' (Diethylenetriamine-N,N,N',N,N-pentaacetic acid, pentetic acid,(Carboxymethyl)imino]bis(ethylenenitrilo)-tetra-acetic acid) is a polyaminopolycarboxylic acid (like EDTA) chelator of metal cations. DTPA wraps around a metal ion by forming up to eight bounds, because each COO- group and and N-center serves a center for chelation. With transition metals the number of bounds is less than eight. The compound is not cell membrane permeable. In general, it chelates multivalent ions stronger than EDTA. | |description='''DTPA''' (Diethylenetriamine-N,N,N',N,N-pentaacetic acid, pentetic acid,(Carboxymethyl)imino]bis(ethylenenitrilo)-tetra-acetic acid) is a polyaminopolycarboxylic acid (like EDTA) chelator of metal cations. DTPA wraps around a metal ion by forming up to eight bounds, because each COO- group and and N-center serves a center for chelation. With transition metals the number of bounds is less than eight. The compound is not cell membrane permeable. In general, it chelates multivalent ions stronger than EDTA. | ||
}} | }} | ||
{{MitoPedia concepts}} | |||
{{MitoPedia methods | |||
|mitopedia method=Fluorometry | |||
}} | |||
{{MitoPedia O2k and high-resolution respirometry}} | |||
{{MitoPedia topics}} | |||
[[File:DTPA structure.gif|right|200px|DTPA structure]] | |||
__TOC__ | __TOC__ | ||
== Application in [[HRR]] == | |||
:::'''Fluorometry:''' | |||
::::* In [[Amplex UltraRed]] assay systems with [[HRP]], there is a spontaneous increase in the UltroxRed fluorescence signal over the experimental time without any biological sample. This high background flux can be related to iron contamination. We have done a set of experiments, where we added DTPA as an iron-chelator into the O2k-chamber during AmR measurements. As a matter of fact, DTPA significantly reduced the high chemical background flux in MiR05 without affecting respiration. | |||
::::* Administration of DTPA into the O2k-chamber is recommended before all other chemicals because the iron chelation of the compound is time-dependent; it takes 10-15 min. | |||
::::* Experiments may also be run without DTPA. | |||
:::'''DTPA''' (C<sub>2</sub>H<sub>2</sub>O<sub>2</sub>); Dr.Ehrenstorfer GmbH (E 13095000) or Sigma Aldrich (D1133); CAS 67-43-6; The commercial powder can be stored at room temperature. The stock solution has to be stored at -20 °C; M: 393.35 g·mol<sup>-1</sup> | |||
::::* '''Solubility:''' It is soluble in water (4,8 g/l at 25 °C), but not readily soluble in ethanol or apolar solvents. | ::::* '''Solubility:''' It is soluble in water (4,8 g/l at 25 °C), but not readily soluble in ethanol or apolar solvents. | ||
::::* '''Stability constant:''' see: [http://www.dojindo.com/Images/Product%20Photo/Chelate_Table_of_Stability_Constants.pdf]: | ::::* '''Stability constant:''' see: [http://www.dojindo.com/Images/Product%20Photo/Chelate_Table_of_Stability_Constants.pdf]: | ||
| Line 33: | Line 38: | ||
|- | |- | ||
|} | |} | ||
::: '''Preparation of the 5 mM stock solution of DTPA:''' | ::: '''Preparation of the 5 mM stock solution of DTPA:''' | ||
::::# Weigh 1.967 mg of DTPA in a 2 mL glass vial. | ::::# Weigh 1.967 mg of DTPA in a 2 mL glass vial. | ||
::::# Dissolve in 500 µL | ::::# Dissolve in 500 µL H<sub>2</sub>O and add 6 µL of 5 M KOH to set the pH. | ||
::::# Heat up the solution up to 33 °C. | ::::# Heat up the solution up to 33 °C. | ||
::::# Add 2 µL 5 M KOH to adjust the pH ~ 8-9. | ::::# Add 2 µL 5 M KOH to adjust the pH ~ 8-9. | ||
::::# Add 500 µL | ::::# Add 500 µL H<sub>2</sub>O to get 5 mM stock solution. | ||
::::# | ::::# Divide into 50 µL portions in Eppendorf tubes. | ||
::::# Store at -20 °C. | |||
:::»'''O2k manual titrations:''' [[MiPNet09.12 O2k-Titrations]] | :::»'''O2k manual titrations:''' [[MiPNet09.12 O2k-Titrations]] | ||
::::* Final concentration in O2k-chamber: 15 µM | ::::* Final concentration in O2k-chamber: 15 µM | ||
::::* Stock solution: 5 mM | ::::* Stock solution: 5 mM | ||
::::* Titration volume: 6 µL | ::::* Titration volume: 6 µL | ||
Revision as of 15:55, 4 April 2024
Description
[[Description::DTPA (Diethylenetriamine-N,N,N',N,N-pentaacetic acid, pentetic acid,(Carboxymethyl)imino]bis(ethylenenitrilo)-tetra-acetic acid) is a polyaminopolycarboxylic acid (like EDTA) chelator of metal cations. DTPA wraps around a metal ion by forming up to eight bounds, because each COO- group and and N-center serves a center for chelation. With transition metals the number of bounds is less than eight. The compound is not cell membrane permeable. In general, it chelates multivalent ions stronger than EDTA.]]
Abbreviation: DTPA
MitoPedia methods: Fluorometry
Application in HRR
- Fluorometry:
- In Amplex UltraRed assay systems with HRP, there is a spontaneous increase in the UltroxRed fluorescence signal over the experimental time without any biological sample. This high background flux can be related to iron contamination. We have done a set of experiments, where we added DTPA as an iron-chelator into the O2k-chamber during AmR measurements. As a matter of fact, DTPA significantly reduced the high chemical background flux in MiR05 without affecting respiration.
- Administration of DTPA into the O2k-chamber is recommended before all other chemicals because the iron chelation of the compound is time-dependent; it takes 10-15 min.
- Experiments may also be run without DTPA.
- DTPA (C2H2O2); Dr.Ehrenstorfer GmbH (E 13095000) or Sigma Aldrich (D1133); CAS 67-43-6; The commercial powder can be stored at room temperature. The stock solution has to be stored at -20 °C; M: 393.35 g·mol-1
- Fluorometry:
- Solubility: It is soluble in water (4,8 g/l at 25 °C), but not readily soluble in ethanol or apolar solvents.
- Stability constant: see: [1]:
Metal DTPA EDTA Ca(II) 10.74 10.96 Mg(II) 9.3 8.69 Fe(II) 16.5 14.3 Fe(III) 28.6 25.1 Mn(II) 15.6 14.04 Zn(II) 18.75 16.5
- Preparation of the 5 mM stock solution of DTPA:
- Weigh 1.967 mg of DTPA in a 2 mL glass vial.
- Dissolve in 500 µL H2O and add 6 µL of 5 M KOH to set the pH.
- Heat up the solution up to 33 °C.
- Add 2 µL 5 M KOH to adjust the pH ~ 8-9.
- Add 500 µL H2O to get 5 mM stock solution.
- Divide into 50 µL portions in Eppendorf tubes.
- Store at -20 °C.
- »O2k manual titrations: MiPNet09.12 O2k-Titrations
- Final concentration in O2k-chamber: 15 µM
- Stock solution: 5 mM
- Titration volume: 6 µL