Difference between revisions of "Pressure"
| Line 18: | Line 18: | ||
</gallery> | </gallery> | ||
== References == | |||
::::* Maxwell JC ( 1867) On the dynamical theory of gases. Phil Trans Royal Soc London 157:49-88. - [[Maxwell 1867 Phil Trans Royal Soc London | »Bioglast link«]] | |||
::::* van't Hoff JH (1901) Osmotic pressure and chemical equilibrium. Nobel Lecture December 13, 1901. - [[Van't Hoff 1901 Nobel Lecture | »Bioglast link«]] | |||
::::* Einstein A (1905) Über die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen. Ann Physik 4, XVII:549-60. - [[Einstein 1905 Ann Physik 549 | »Bioglast link«]] | |||
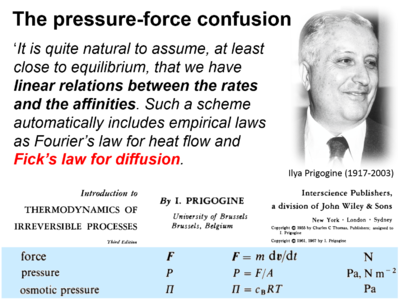
::::* Prigogine I (1967) Introduction to thermodynamics of irreversible processes. Interscience New York, 3rd ed:147pp. - [[Prigogine 1967 Interscience | »Bioglast link«]] | |||
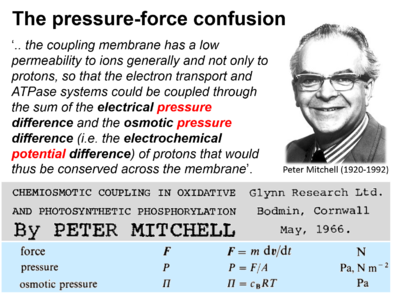
::::* Mitchell P (1966) Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biochim Biophys Acta Bioenergetics 1807 (2011):1507-38. - [[Mitchell 2011 Biochim Biophys Acta | »Bioglast link«]] | |||
{{MitoPedia concepts | {{MitoPedia concepts | ||
|mitopedia concept=MiP concept, Ergodynamics | |mitopedia concept=MiP concept, Ergodynamics | ||
}} | }} | ||
Revision as of 13:06, 16 September 2018
Description
Pressure [Pa = J·m-3] is the concentration of the force at the point of action. More generally, pressure is the force times concentration at the interphase of interaction.
In addition to mechanical pressure, hydrostatic pressure, barometric pressure, gas pressure (oxygen pressure), isomorphic pressures are distinguished as osmotic pressure, diffusion pressure, reaction pressure, and even electric pressure. In ergodynamics, the pressure in a transformation, ΔtrΠ, is the product of free activity times force, ΔtrΠ = αtr·ΔtrF [mol·m-3 · J·mol-1 = J·m-3 = Pa].
In the classical physicochemical literature, there is some confusion between the terms force and pressure: "This force is called the pressure of the gas" by Maxwell (1867); "This pressure is osmotic pressure. .. Osmotic forces are in fact .." by van't Hoff 1901; "Pressure-forces" by Einstein (1905); presentation of Fick's law of diffusion (which represents a flux-pressure relationship) as a flux-force relationship by Prigogine (1967).
Abbreviation: P, p, Π
Reference: Gnaiger 1989 Energy Transformations; Gnaiger 2017 MiP2017
Comunicated by Erich Gnaiger 2018-09-16
References
- Maxwell JC ( 1867) On the dynamical theory of gases. Phil Trans Royal Soc London 157:49-88. - »Bioglast link«
- van't Hoff JH (1901) Osmotic pressure and chemical equilibrium. Nobel Lecture December 13, 1901. - »Bioglast link«
- Einstein A (1905) Über die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen. Ann Physik 4, XVII:549-60. - »Bioglast link«
- Prigogine I (1967) Introduction to thermodynamics of irreversible processes. Interscience New York, 3rd ed:147pp. - »Bioglast link«
- Mitchell P (1966) Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biochim Biophys Acta Bioenergetics 1807 (2011):1507-38. - »Bioglast link«
MitoPedia concepts:
MiP concept,
Ergodynamics