Difference between revisions of "Mole"
From Bioblast
(Created page with "{{MitoPedia |abbr=mol |description=The mole [mol] is the SI base unit for the amount of substance of a system that contains 6.02214076 x 1023 specified elementary...") |
|||
| (8 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{MitoPedia | {{MitoPedia | ||
|abbr=mol | |abbr=mol | ||
|description=The mole [mol] is the SI base unit for the [[amount |amount of substance]] of a system that contains 6. | |description=The mole [mol] is the SI base unit for the [[amount |amount of substance]] of a system that contains 6.02214076·10<sup>23</sup> specified elementary entities (see [[Avogadro constant]]). The elementary entities must be specified and may be atoms, molecules, ions, electrons, other particles, or specified groups of such particles. | ||
|info=[[ | |info=[[Bureau International des Poids et Mesures 2019 The International System of Units (SI)]], [[Gnaiger MitoFit Preprints 2020.4]] | ||
}} | }} | ||
== The redefined international system of units == | |||
[[File:SI-units.png|left|120px]] | |||
:::: Since 2019-05-20, the definition of the mole is (from [https://www.euramet.org/si-redefinition/countdown-si-redefinition/the-mole/ EURAMET]): | |||
:::::: The mole, symbol mol, is the SI unit of amount of substance. One mole contains exactly 6.022 140 76 × 10<sup>23</sup> elementary entities. This number is the fixed numerical value of the [[Avogadro constant]], ''N''<sub>A</sub>, when expressed in the unit mol<sup>−1</sup> and is called the Avogadro number. | |||
:::::: The [[amount of substance]], symbol ''n'', of a system is a measure of the number of specified elementary entities. An elementary entity may be an atom, a molecule, an ion, an electron, any other particle or specified group of particles. | |||
:::: From 1971 until 2019-05-20, the definition of the mole has been: | |||
:::::# The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilograms of carbon 12; its symbol is "mol". | |||
:::::# When the mole is used, the elementary entities must be specified and may be atoms, molecules, ions, electrons, other particles, or specified groups of such particles. | |||
{{Template:Base quantities and count}} | |||
{{Keywords: SI base units}} | |||
{{Keywords: Concentration and pressure}} | |||
{{MitoPedia concepts | {{MitoPedia concepts | ||
|mitopedia concept=Ergodynamics | |mitopedia concept=Ergodynamics | ||
}} | }} | ||
Latest revision as of 15:45, 18 August 2021
Description
The mole [mol] is the SI base unit for the amount of substance of a system that contains 6.02214076·1023 specified elementary entities (see Avogadro constant). The elementary entities must be specified and may be atoms, molecules, ions, electrons, other particles, or specified groups of such particles.
Abbreviation: mol
Reference: Bureau International des Poids et Mesures 2019 The International System of Units (SI), Gnaiger MitoFit Preprints 2020.4
The redefined international system of units
- Since 2019-05-20, the definition of the mole is (from EURAMET):
- The mole, symbol mol, is the SI unit of amount of substance. One mole contains exactly 6.022 140 76 × 1023 elementary entities. This number is the fixed numerical value of the Avogadro constant, NA, when expressed in the unit mol−1 and is called the Avogadro number.
- The amount of substance, symbol n, of a system is a measure of the number of specified elementary entities. An elementary entity may be an atom, a molecule, an ion, an electron, any other particle or specified group of particles.
- From 1971 until 2019-05-20, the definition of the mole has been:
- The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilograms of carbon 12; its symbol is "mol".
- When the mole is used, the elementary entities must be specified and may be atoms, molecules, ions, electrons, other particles, or specified groups of such particles.
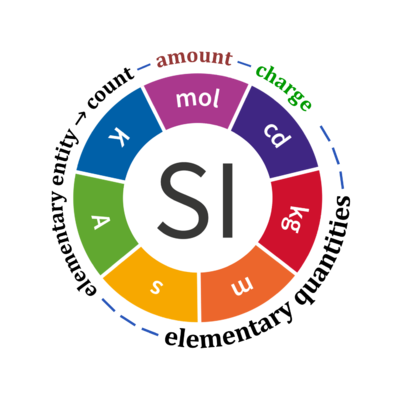
Quantity Symbol for quantity Q Symbol for dimension Name of abstract unit uQ Symbol for unit uQ [*] elementary entity *,$ UX U elementary unit x count *,$ NX = N·UX X elementary unit x amount of substance *,§ nX = NX·NA-1 N mole mol charge *,€ Qel = zX·e·NX I·T coulomb C = A·s length l L meter m mass m M kilogram kg time t T second s electric current I I ampere A thermodynamic temperature T Θ kelvin K luminous intensity Iv J candela cd
- [*] SI units, except for the canonical 'elementary unit' [x]. The following footnotes are canonical comments, related to iconic symbols.
- * For the elementary quantities NX, nX, and Qel, the entity-type X of the elementary entity UX has to be specified in the text and indicated by a subscript: nO2; Nce; Qel.
- $ Count NX equals the number of elementary entities UX. In the SI, the quantity 'count' is explicitly considered as an exception: "Each of the seven base quantities used in the SI is regarded as having its own dimension. .. All other quantities, with the exception of counts, are derived quantities" (Bureau International des Poids et Mesures 2019 The International System of Units (SI)). An elementary entity UX is a material unit, it is not a count (UX is not a number of UX). NX has the dimension X of a count and UX has the dimension U of an elementary entity; both quantities have the same abstract unit, the 'elementary unit' [x].
- § Amount nX is an elementary quantity, converting the elementary unit [x] into the SI base unit mole [mol] using the Avogadro constant NA.
- € Charge is a derived SI quantity. Charge is an elementary quantity, converting the elementary unit [x] into coulombs [C] using the elementary charge e, or converting moles [mol] into coulombs [C] using the Faraday constant F. zX is the charge number per elementary entity UX, which is a constant for any defined elementary entity UX. Qel = zX·F·nX
- Bioblast links: SI base units - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Entity, count, and number, and SI base quantities / SI base units
Quantity name Symbol Unit name Symbol Comment elementary UX elementary unit [x] UX, UB; [x] not in SI count NX elementary unit [x] NX, NB; [x] not in SI number N - dimensionless = NX·UX-1 amount of substance nB mole [mol] nX, nB electric current I ampere [A] A = C·s-1 time t second [s] length l meter [m] SI: metre mass m kilogram [kg] thermodynamic temperature T kelvin [K] luminous intensity IV candela [cd]
- Fundamental relationships
- » Avogadro constant NA
- » Boltzmann constant k
- » elementary charge e
- » Faraday constant F
- » gas constant R
- » electrochemical constant f
- Fundamental relationships
- SI and related concepts
- Bioblast links: Concentration and pressure - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Concentration
- » Volume
- » Activity
- » Concentration
- » Density
- » Mole
- » Molar mass
- Concentration
- Pressure
- Solubility = concentration/pressure
- General
- » Boltzmann constant
- » Energy
- » Force
- » Gas constant
- » Work
- General
- Related keyword lists
MitoPedia concepts:
Ergodynamics