Difference between revisions of "MitoFit-Medical device"
Laner Verena (talk | contribs) |
Laner Verena (talk | contribs) |
||
| Line 20: | Line 20: | ||
::::* [[ISO 17511:2003 In vitro diagnostic medical devices]] | ::::* [[ISO 17511:2003 In vitro diagnostic medical devices]] | ||
::::* [[MitoFit-O2k#Workpackages|Back to | ::::* [[MitoFit-O2k#Workpackages|Back to overview]] | ||
Revision as of 20:59, 21 February 2016
MitoFit-Medical device
- The project MitoFit highlights the benefits of mitochondrial fitness.
The O2k from research instrument to medical device
- Abstract
- The legal provisions and their impact on the instrument will be collected and evaluated. An action plan will be developed, describing all necessary steps on the way for the O2k to become a medical device. The most effective but also most expensive marketing strategy would be to seek approval as a medical device simultaneously with a CE Mark and FDA clearance for the O2k, since 44% and 24% of our present research instruments are placed in the EU and US, respectively. Regulatory risks need to be considered for the launch of the O2k as a medical device, versus the risk of loosing a market in the EU, US or other countries when audit requirements become more stringent even in the field of mitochondrial research linked to human health and disease.
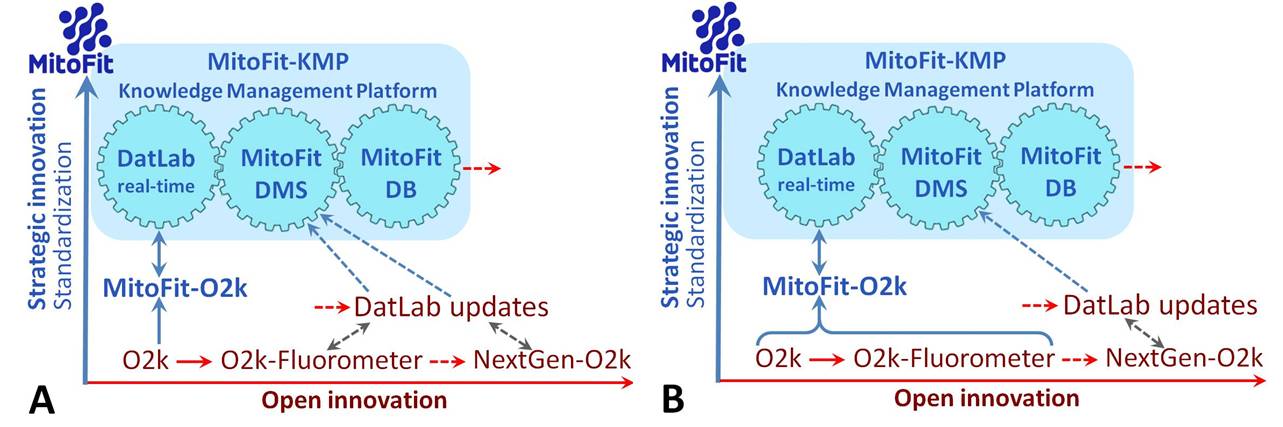
Extending previous and ongoing ‘Open innovation’ (red), the concept of ‘Strategic innovation’ is implemented into the MitoFit project towards standardization of the O2k as a clinical device for diagnostic assays with the integrated MitoFit-Knowledge Management Platform (MitoFit-KMP). Two strategies have to be evaluated: Scenario A: Based on long-term experience, the O2k-Core (O2k) will be the sole target of standardization as a medical device. The present O2k-Fluorometer will be subject to further development according to the concept of Open innovation. Scenario B: Within the 3-year period of the MitoFit project, the O2k-Fluorometer and related protocols will be sufficiently standardized to be included into the MitoFit-O2k concept as a medical device.
- Links
- Websites CE and FDA approval: FDA: In Vitro Diagnostics