Difference between revisions of "Gas constant"
From Bioblast
| (6 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{MitoPedia | {{MitoPedia | ||
|abbr=''R'' [J·mol<sup>-1</sup>·K<sup>-1</sup>] | |abbr=''R'' [J·mol<sup>-1</sup>·K<sup>-1</sup>] | ||
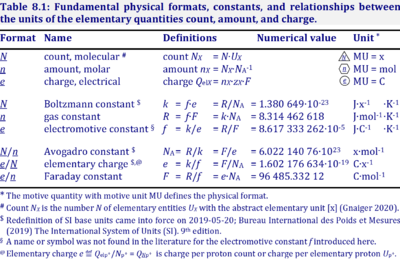
|description=[[File:Table Physical constants.png|left|400px|thumb|]] | |description=[[File:Table Physical constants.png|left|400px|thumb|]] The '''gas constant''', ''R'' = 8.314462618 J·mol<sup>-1</sup>·K<sup>-1</sup>, has the SI unit for energy per amount per temperature. ''R'' is primarily known from the ideal gas equation, ''pV'' = ''nRT'' or ''p'' = ''cRT''. Therefore, ''RT'' is the ratio of pressure ''p'' and concentration ''c''. | ||
''R'' = ''f''·''F'', the [[electrochemical constant]] ''f'' times the [[Faraday constant]] ''F''. | |||
''R'' = ''k''·''N''<sub>A</sub>, the [[Boltzmann constant]] ''k'' times the [[Avogadro constant]] ''N''<sub>A</sub>. | |||
|info=[[Gnaiger 2020 BEC MitoPathways]] | |||
}} | }} | ||
<br> | |||
<br> | |||
<br> | |||
<br> | |||
[[File:Table RT.png|right|300px|thumb|]] | |||
Communicated by [[Gnaiger E]] (2018-10-18) last update 2020-11-24 | |||
<br> | |||
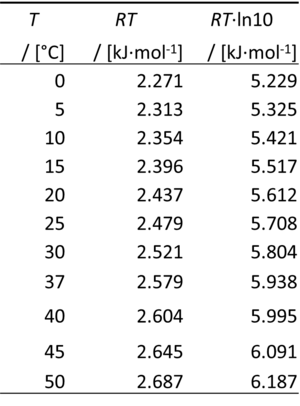
:::: 0 °C = 273.15 K | |||
:::: ln(10) = 2.302585093 | |||
::::» ''See'': [[Chemical potential]] | ::::» ''See'': [[Chemical potential]] | ||
== References == | == References == | ||
::::# Bureau International des Poids et Mesures (2019) The International System of Units (SI). 9th edition:117-216 ISBN 978-92-822-2272-0. - [[Bureau International des Poids et Mesures 2019 The International System of Units (SI) |»Bioblast link«]] | |||
::::# Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. - [[Gnaiger 2020 BEC MitoPathways |»Bioblast link«]] | |||
: | {{Keywords: SI base units}} | ||
: | {{Keywords: Concentration and pressure}} | ||
{{MitoPedia concepts | {{MitoPedia concepts | ||
|mitopedia concept=Ergodynamics | |mitopedia concept=Ergodynamics | ||
}} | }} | ||
Latest revision as of 17:00, 26 October 2022
Description
The gas constant, R = 8.314462618 J·mol-1·K-1, has the SI unit for energy per amount per temperature. R is primarily known from the ideal gas equation, pV = nRT or p = cRT. Therefore, RT is the ratio of pressure p and concentration c.
R = f·F, the electrochemical constant f times the Faraday constant F.
R = k·NA, the Boltzmann constant k times the Avogadro constant NA.
Abbreviation: R [J·mol-1·K-1]
Reference: Gnaiger 2020 BEC MitoPathways
Communicated by Gnaiger E (2018-10-18) last update 2020-11-24
- 0 °C = 273.15 K
- ln(10) = 2.302585093
- » See: Chemical potential
References
- Bureau International des Poids et Mesures (2019) The International System of Units (SI). 9th edition:117-216 ISBN 978-92-822-2272-0. - »Bioblast link«
- Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. - »Bioblast link«
- Bioblast links: SI base units - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Entity, count, and number, and SI base quantities / SI base units
Quantity name Symbol Unit name Symbol Comment elementary UX elementary unit [x] UX, UB; [x] not in SI count NX elementary unit [x] NX, NB; [x] not in SI number N - dimensionless = NX·UX-1 amount of substance nB mole [mol] nX, nB electric current I ampere [A] A = C·s-1 time t second [s] length l meter [m] SI: metre mass m kilogram [kg] thermodynamic temperature T kelvin [K] luminous intensity IV candela [cd]
- Fundamental relationships
- » Avogadro constant NA
- » Boltzmann constant k
- » elementary charge e
- » Faraday constant F
- » gas constant R
- » electrochemical constant f
- Fundamental relationships
- SI and related concepts
- Bioblast links: Concentration and pressure - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Concentration
- » Volume
- » Activity
- » Concentration
- » Density
- » Mole
- » Molar mass
- Concentration
- Pressure
- Solubility = concentration/pressure
- General
- » Boltzmann constant
- » Energy
- » Force
- » Gas constant
- » Work
- General
- Related keyword lists
MitoPedia concepts:
Ergodynamics