Vesga 2021 Med Chem Res
| Vesga LC, Silva AMP, Bernal CC, Mendez-Sánchez SC, Bohórquez ARR (2021) Tetrahydroquinoline/4,5-dihydroisoxazole hybrids with a remarkable effect over mitochondrial bioenergetic metabolism on melanoma cell line B16F10. Med Chem Res 30:2127–43. https://doi.org/10.1007/s00044-021-02796-5. |
» Springer
Vesga LC, Silva AMP, Bernal CC, Mendez-Sanchez SC, Bohorquez ARR (2021) Med Chem Res
Abstract: Metastatic melanoma, unlike early-stage melanomas, requires significant interventions with a poor prognosis. In consequence, we evaluated the effect of three tetrahydroquinoline/4,5-dihydroisoxazole hybrids (CB35, CB46, and CB50) on cellular respiration in murine melanoma cells (B16F10). Additionally, the impact of these compounds against the main antioxidant enzymes and the electron transport on the mitochondrial respiratory chain also was determined. The effect on cellular respiration indicates that compound CB35 (10 µM) inhibits the electron transport through the mitochondrial respiratory chain after 24 h of treatment. This inhibition could be related to the transport of electrons to cytochrome c due to the decreasing activity in the NADH cytochrome c reductase at 1 µM in disrupted mitochondria. On the other hand, compounds CB46 and CB50 generate an uncoupling effect on oxidative phosphorylation by increasing oxygen consumption in the LEAK state (10 µM, 24 h), as well as the increase in state 4 in the mitochondrial bioenergetics. In all cases, molecular hybrids increase ATPase activity in coupled mitochondria, suggesting an uncoupled effect. All hybrids disrupt the redox system in B16F10, and the glycolytic pathway by inhibiting hexokinase and pyruvate kinase activities.
• Bioblast editor: Gnaiger E
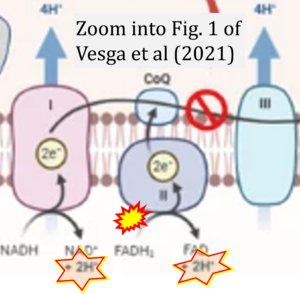
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.