Protti 2006 Crit Care
| Protti A, Singer M (2006) Bench-to-bedside review: potential strategies to protect or reverse mitochondrial dysfunction in sepsis-induced organ failure. Crit Care 10:228. doi: 10.1186/cc5014 |
Protti A, Singer M (2006) Crit Care
Abstract: The pathogenesis of sepsis-induced multiple organ failure may crucially depend on the development of mitochondrial dysfunction and consequent cellular energetic failure. According to this hypothesis, interventions aimed at preventing or reversing mitochondrial damage may have major clinical relevance, although the timing of such interventions will be critical to both ensuring benefit and avoiding harm. Early correction of tissue hypoxia, strict control of glycaemia, and modulation of oxidative and nitrosative stress may afford protection during the initial, acute systemic inflammatory response. The regulated induction of a hypometabolic state resembling hibernation may protect the cells from dying once energy failure has developed, allowing the possibility of functional recovery. Repair of damaged organelles through stimulation of mitochondrial biogenesis and reactivation of cellular metabolism may accelerate resolution of the multiple organ failure syndrome.
• Bioblast editor: Gnaiger E
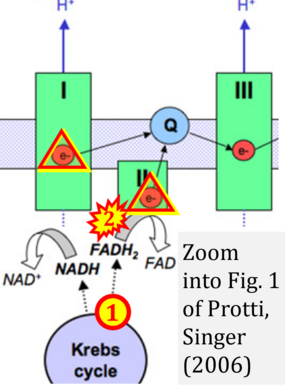
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.
Labels:
Enzyme: Complex II;succinate dehydrogenase