Massoz 2017 Microbiology Monographs
| Massoz S, Cardol P, González-Halphen D, Remacle C (2017) Mitochondrial bioenergetics pathways in Chlamydomonas. In: Hippler M (ed) Chlamydomonas: Molecular genetics and physiology. Microbiology Monographs 30:59-95. Springer, Cham. https://doi.org/10.1007/978-3-319-66365-4_3. |
» [Microbiology Monographs Springer link]
Massoz S, Cardol P, Gonzalez-Halphen D, Remacle C (2017) Microbiology Monographs
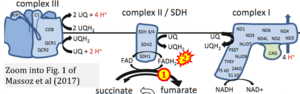
Abstract: Mitochondrion is the site where the Krebs cycle and oxidative phosphorylation (OXPHOS) take place. After a brief overview of Krebs cycle and acetate metabolism in Chlamydomonas, this chapter focuses on OXPHOS components. OXPHOS is composed of five major multiprotein complexes: NADH:ubiquinone oxidoreductase (complex I), succinate dehydrogenase (complex II), ubiquinone:cytochrome c oxidoreductase (complex III), cytochrome c oxidase (complex IV), and ATP synthase. Three complexes (complexes I, III, and IV) pump protons from the matrix to the intermembrane space (IMS) and build a gradient which is used by ATP synthase to produce ATP. In Chlamydomonas and other eukaryotes, proteins forming these complexes have a dual genetic origin. A few proteins, mostly hydrophobic polypeptides, are mitochondrion-encoded, while the vast majority are nucleus-encoded and imported from the cytoplasm. Here we will review our current knowledge about these complexes.
• Bioblast editor: Gnaiger E
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Labels:
Chlamydomonas