Manickam 2022 J Control Release
| Manickam DS (2022) Delivery of mitochondria via extracellular vesicles - a new horizon in drug delivery. J Control Release 343:400-7. https://doi.org/10.1016/j.jconrel.2022.01.045 |
Manickam DS (2022) J Control Release
Abstract: The field of drug delivery has made tremendous advances in increasing the therapeutic potential of a variety of drug candidates spanning from small molecules to large molecular biologics such as nucleic acids, proteins, etc. Extracellular vesicles (EVs) are mediators of intercellular communication and carry a rich cocktail of innate cargo including lipids, proteins and nucleic acids. EVs are a promising class of natural, cell-derived carriers for drug delivery. EVs of particle diameters <200 nm are referred to as small EVs (sEVs) and medium-to-larger particles of diameters >200 nm are referred to as m/lEVs. The m/lEVs naturally incorporate mitochondria during their biogenesis. In this Oration, I will discuss the potential of m/lEVs as carriers for the delivery of healthy and functional mitochondria. Mitochondrial damage and dysfunction play a causal role in multiple pathologies such as neurodegenerative diseases, cardiovascular and metabolic diseases-suggesting that m/lEV-mediated mitochondria delivery can be of broad biomedical significance. A major advantage of harnessing m/lEVs is that the delivered mitochondria are capable of using endogenous mechanisms for repairing the cellular damage. I will highlight the delivery potential of m/lEVs based on the studies we have conducted so far and discuss unaddressed issues towards their development as a novel class of mitochondria carriers.
• Bioblast editor: Gnaiger E
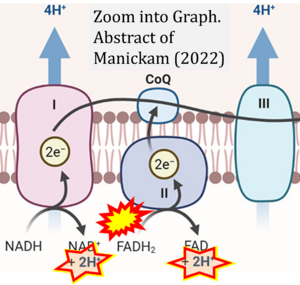
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.