Fahimi 2022 Trends in Chemistry
| Fahimi P, Matta CF (2022) The hot mitochondrion paradox: reconciling theory and experiment. Trends in Chemistry 4:4-20. https://doi.org/10.1016/j.trechm.2021.10.005. |
Fahimi P, Matta CF (2022) Trends in Chemistry
Abstract: Experiments by Chrétien and co-workers suggest that mitochondria are 10oC hotter than their surroundings. Steady-state theoretical estimates place this difference at a maximum of 10-5 oC. This million-fold disagreement may be called the “hot mitochondrion paradox”. It is suggested that every proton translocated via ATP synthase sparks a picosecond temperature-difference spike of the order of magnitude measured by Chrétien et al. Time averaging of these spikes recovers the theoretical value. Further, a temporal and spatial superposition of the fluorescence intensity of a very large number of molecular thermometer molecules in the sample can give the appearance of a steady signal. The inner mitochondrial membrane appears to be flanked by temperature differences fluctuating in time and along the membrane’s surface, with “hot” and “cold” spots as ultrashort temperature spikes.
• Bioblast editor: Gnaiger E
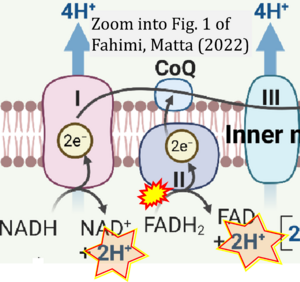
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.