Bellance 2009 Front Biosci (Landmark Ed)
| Bellance N, Lestienne P, Rossignol R (2009) Mitochondria: from bioenergetics to the metabolic regulation of carcinogenesis. Front Biosci (Landmark Ed) 14:4015-34. doi: 10.2741/3509 |
Bellance N, Lestienne P, Rossignol R (2009) Front Biosci (Landmark Ed)
Abstract: In this review, we discuss the concept of metabolic remodeling and signaling in tumors, specifically the various metabolites that participate in the regulation of gene expression in cancer cells. In particular, pyruvate, oxaloacetate, succinate and fumarate, four mitochondrial metabolites, activate genes relevant for tumor progression. When the balance between glycolysis and oxidative phosphorylation is altered, these metabolites accumulate in the cytoplasm and regulate the activity of the Hypoxia Inducible Factor 1alpha (HIF-1alpha). HIF is one of the main factors that orchestrate the metabolic switch observed during oncogenesis. There is also an important role for lactate, fructose 1-6 bisphosphate or citrate that leads to the diversion of glucose metabolites to anabolism. In addition reactive oxygen species, which are produced by the respiratory chain, could serve as an endogenous source of DNA-damaging agents to promote genetic instability. Accordingly, several mitochondrial DNA mutations were reported in tumors, and the construction of cybrids recently demonstrated their role in the control of tumor progression.
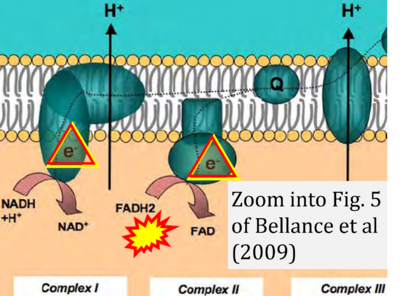
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.
Labels: Pathology: Cancer
Enzyme: Complex II;succinate dehydrogenase