Aye 2022 Am J Obstet Gynecol
| Aye ILMH, Aiken CE, Charnock-Jones DS, Smith GCS (2022) Placental energy metabolism in health and disease-significance of development and implications for preeclampsia. Am J Obstet Gynecol 226:S928-44. https://doi.org/10.1016/j.ajog.2020.11.005 |
Aye ILMH, Aiken CE, Charnock-Jones DS, Smith GCS (2022) Am J Obstet Gynecol
Abstract: The placenta is a highly metabolically active organ fulfilling the bioenergetic and biosynthetic needs to support its own rapid growth and that of the fetus. Placental metabolic dysfunction is a common occurrence in preeclampsia although its causal relationship to the pathophysiology is unclear. At the outset, this may simply be seen as an "engine out of fuel." However, placental metabolism plays a vital role beyond energy production and is linked to physiological and developmental processes. In this review, we discuss the metabolic basis for placental dysfunction and propose that the alterations in energy metabolism may explain many of the placental phenotypes of preeclampsia such as reduced placental and fetal growth, redox imbalance, oxidative stress, altered epigenetic and gene expression profiles, and the functional consequences of these aberrations. We propose that placental metabolic reprogramming reflects the dynamic physiological state allowing the tissue to adapt to developmental changes and respond to preeclampsia stress, whereas the inability to reprogram placental metabolism may result in severe preeclampsia phenotypes. Finally, we discuss common tested and novel therapeutic strategies for treating placental dysfunction in preeclampsia and their impact on placental energy metabolism as possible explanations into their potential benefits or harm.
• Bioblast editor: Gnaiger E
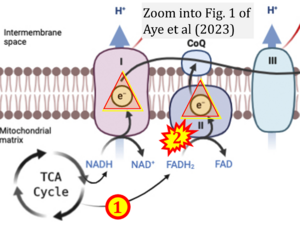
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.