Alston 2017 J Pathol
| Alston CL, Rocha MC, Lax NZ, Turnbull DM, Taylor RW (2017) The genetics and pathology of mitochondrial disease. J Pathol 241:236-50. doi: 10.1002/path.4809 |
Alston CL, Rocha MC, Lax NZ, Turnbull DM, Taylor RW (2017) J Pathol
Abstract: Mitochondria are double-membrane-bound organelles that are present in all nucleated eukaryotic cells and are responsible for the production of cellular energy in the form of ATP. Mitochondrial function is under dual genetic control - the 16.6-kb mitochondrial genome, with only 37 genes, and the nuclear genome, which encodes the remaining ∼1300 proteins of the mitoproteome. Mitochondrial dysfunction can arise because of defects in either mitochondrial DNA or nuclear mitochondrial genes, and can present in childhood or adulthood in association with vast clinical heterogeneity, with symptoms affecting a single organ or tissue, or multisystem involvement. There is no cure for mitochondrial disease for the vast majority of mitochondrial disease patients, and a genetic diagnosis is therefore crucial for genetic counselling and recurrence risk calculation, and can impact on the clinical management of affected patients. Next-generation sequencing strategies are proving pivotal in the discovery of new disease genes and the diagnosis of clinically affected patients; mutations in >250 genes have now been shown to cause mitochondrial disease, and the biochemical, histochemical, immunocytochemical and neuropathological characterization of these patients has led to improved diagnostic testing strategies and novel diagnostic techniques. This review focuses on the current genetic landscape associated with mitochondrial disease, before focusing on advances in studying associated mitochondrial pathology in two, clinically relevant organs - skeletal muscle and brain. © 2016 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
• Bioblast editor: Gnaiger E
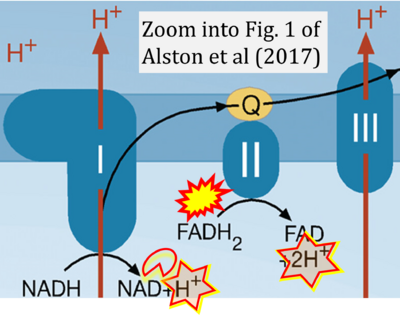
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Labels:
Enzyme: Complex II;succinate dehydrogenase