Semantic search
| Term | Abbreviation | Description |
|---|---|---|
| Hydrogen sulfide | H2S | Hydrogen sulfide (H2S) is involved in signaling and may have have further biological importance. |
| Hydrogenion flux | JH+ | Volume-specific hydrogenion flux or H+ flux is measured in a closed system as the time derivative of H+ concentration, expressed in units [pmol·s-1·mL-1]. H+ flux can be measured in an open system at steady state, when any acidification of the medium is compensated by external supply of an equivalent amount of base. The extracellular acidification rate (ECAR) is the change of pH in the incubation medium over time, which is zero at steady state. Volume-specific H+ flux is comparable to volume-specific oxygen flux [pmol·s-1·mL-1], which is the (negative) time derivative of oxygen concentration measured in a closed system, corrected for instrumental and chemical background. pH is the negative logarithm of hydrogen ion activity. Therefore, ECAR is of interest in relation to acidification issues in the incubation buffer or culture medium. The physiologically relevant metabolic H+ flux, however, must not be confused with ECAR. |
| Hydron | H+ | Hydron is the general name for the cation H+ used without regard to the nuclear mass of the hydrogen entity (H is the hydro group), either for H in its natural abundance or without distinction between the isotopes. |
| Hydronium ion | H3O+ | H+ forms the hydronium ion H3O+, which in turn is further solvated by water molecules in clusters such as H5O2+ and H9O4+. |
| Hydroxycinnamate | Hci | Hydroxycinnamate (alpha-cyano-4-hydroxycinnamic acid) is an inhibitor of the pyruvate carrier (0.65 mM). Above 10 mM pyruvate, hydroxycinnamate cannot inhibit respiration from pyruvate, since the weak pyruvic acid can pass the inner mt-membrane in non-dissociated form. |
| Hyperoxia | hyperox | Hyperoxia is defined as environmental oxygen pressure above the normoxic reference level. Cellular and intracellular hyperoxia is imposed on isolated cells and isolated mitochondria at air-level oxygen pressures which are higher compared to cellular and intracellular oxygen pressures under tissue conditions in vivo. Hyperoxic conditions may impose oxidative stress and may increase maximum aerobic performance. |
| Hypoxia | hypox | Hypoxia (hypox) is defined in respiratory physiology as the state when insufficient O2 is available for respiration, compared to environmental hypoxia defined as environmental oxygen pressures below the normoxic reference level. Three major categories of hypoxia are (1) environmental hypoxia, (2) physiological tissue hypoxia in hyperactivated states (e.g. at VO2max) with intracellular oxygen demand/supply balance at steady state in tissues at environmental normoxia, compared to tissue normoxia in physiologically balanced states, and (3) pathological tissue hypoxia including ischemia and stroke, anaemia, chronic heart disease, chronic obstructive pulmonary disease, severe COVID-19, and obstructive sleep apnea. Pathological hypoxia leads to tissue hypoxia and heterogenous intracellular anoxia. Clinical oxygen treatment ('environmental hyperoxia') may not or only partially overcome pathological tissue hypoxia. |
| IRDiRC | IRDiRC |  The International Rare Diseases Research Consortium (IRDiRC) teams up researchers and organizations investing in rare diseases research in order to achieve two main objectives by the year 2020, namely to deliver 200 new therapies for rare diseases and means to diagnose most rare diseases. The International Rare Diseases Research Consortium (IRDiRC) teams up researchers and organizations investing in rare diseases research in order to achieve two main objectives by the year 2020, namely to deliver 200 new therapies for rare diseases and means to diagnose most rare diseases. |
| ISO 10012:2003 Measurement management systems | ISO 10012:2003 | ISO 10012:2003 Measurement management systems — Requirements for measurement processes and measuring equipment: An effective measurement management system ensures that measuring equipment and measurement processes are fit for their intended use and is important in achieving product quality objectives and managing the risk of incorrect measurement results. The objective of a measurement management system is to manage the risk that measuring equipment and measurement processes could produce incorrect results affecting the quality of an organization’s product. The methods used for the measurement management system range from basic equipment verification to the application of statistical techniques in the measurement process control. |
| ISO 13528:2015 Statistical methods for use in proficiency testing by interlaboratory comparison | ISO 13528:2015 | ISO 13528:2015 Statistical methods for use in proficiency testing by interlaboratory comparison: Proficiency testing involves the use of interlaboratory comparisons to determine the performance of participants (which may be laboratories, inspection bodies, or individuals) for specific tests or measurements, and to monitor their continuing performance. There are a number of typical purposes of proficiency testing ISO/IEC 17043:2010. These include the evaluation of laboratory performance, the identification of problems in laboratories, establishing effectiveness and comparability of test or measurement methods, the provision of additional confidence to laboratory customers, validation of uncertainty claims, and the education of participating laboratories. The statistical design and analytical techniques applied must be appropriate for the stated purpose(s). |
| ISO 15189:2012 Medical laboratories — Particular requirements for quality and competence | ISO 15189:2012 | ISO 15189:2012 Medical laboratories — Particular requirements for quality and competence: This International Standard is for use by medical laboratories in developing their quality management systems and assessing their own competence, and for use by accreditation bodies in confirming or recognising the competence of medical laboratories. While this International Standard is intended for use throughout the currently recognised disciplines of medical laboratory services, those working in other services and disciplines could also find it useful and appropriate. |
| ISO 17511:2003 In vitro diagnostic medical devices | ISO 17511:2003 | ISO 17511:2003 In vitro diagnostic medical devices -- Measurement of quantities in biological samples -- Metrological traceability of values assigned to calibrators and control materials: For measurements of quantities in laboratory medicine, it is essential that the quantity is adequately defined and that the results reported to the physicians or other health care personel and patients are adequately accurate (true and precise) to allow correct medical interpretation and comparability over time and space. |
| ISO 9001:2015 Quality management systems - requirements | ISO 9001:2015 | ISO 9001:2015 Quality management systems - requirements: The adoption of a quality management system is a strategic decision for an organization that can help to improve its overall performance and provide a sound basis for sustainable development initiatives. Consistently meeting requirements and addressing future needs and expectations poses a challenge for organizations in an increasingly dynamic and complex environment. To achieve this objective, the organization might find it necessary to adopt various forms of improvement in addition to correction and continual improvement, such as breakthrough change, innovation and re-organization. |
| ISO/IEC 17025:2005 Competence of testing and calibration laboratories | ISO/IEC 17025:2005 | ISO/IEC 17025:2005 General requirements for the competence of testing and calibration laboratories: The use of this International Standard will facilitate cooperation between laboratories and other bodies, and assist in the exchange of information and experience, and in the harmonization of standards and procedures. This International Standard specifies the general requirements for the competence to carry out tests and/or calibrations, including sampling. It covers testing and calibration performed using standard methods, non-standard methods, and laboratory-developed methods. |
| ISO/IEC 17043:2010 General requirements for proficiency testing | ISO/IEC 17043:2010 | ISO/IEC 17043:2010 Conformity assessment — General requirements for proficiency testing: The use of interlaboratory comparisons is increasing internationally. This International Standard provides a consistent basis to determine the competence of organizations that provide proficiency testing. |
| Illumination | F10 | The chambers of the Oroboros O2k are illuminated by an internal LED. The illumination is switched on and off in DatLab during the experiment by pressing [F10]. This illumination must be distinguished from light introduced into the chambers by LEDs for the purpose of spectrophotometric and fluorometric measurements. For these, the internal illumination must be switched off. |
| Illumination on/off | F10 | The illumination in both chambers is switched on/off. |
| Impact factor | IF | Impact factor is a measure of a scientific journal's citations per publication. The Journal Citation Reports, maintained by Clarivate Analytics, provides the calculated impact factors. The IF is frequently used as an indicator of a journal's importance or prestige, which is nowadays increasingly contested. |
| Improvement score | RIS | The relative improvement score, RIS, provides a measure of improvement of a trait from a value measured at baseline, B, to a value measured after treatment, T, expressing the total improvement, T-B, in relation to the theoretical scope of improvement and the level of the trait observed at baseline. 'RIS incorporates the concept of diminishing returns and consideres maintaining a high value of a trait as an improvement relative to the potential loss. |
| In vitro diagnostic medical device | IVD | A medical device is an in vitro diagnostic medical device (IVD) if it is a reagent, calibrator, control material, kit, specimen receptacle, software, instrument, apparatus, equipment or system, whether used alone or in combination with other diagnostic goods for in vitro use. |
| Inorganic phosphate | Pi | Inorgnic phosphate (Pi) is a salt of phosphoric acid. In solution near physiological pH, the species HPO42- and H2PO4- dominate. See also: Phosphate carrier (Pic). |
| Internal flow | Ii [MU·s-1] | Within the system boundaries, irreversible internal flows, Ii,—including chemical reactions and the dissipation of internal gradients of heat and matter—contribute to internal entropy production, diS/dt. In contrast, external flows, Ie, of heat, work, and matter proceed reversibly across the system boundaries (of zero thickness). Flows are expressed in various formats per unit of time, with corresponding motive units [MU], such as chemical [mol], electrical [C], mass [kg]. Flow is an extensive quantity, in contrast to flux as a specific quantity. |
| Internal-energy | U [J] | Internal-energy, U [J], can neither be destroyed nor created (first law of thermodynamics: diU/dt = 0). Note that internal (subscript i), as opposed to external (subscript e), must be distinguished from "internal-energy", U, which contrasts with "Helmholtz energy", A, as enthalpy, H, contrasts with Gibbs energy, G. |
| International Mito Patients (IMP) | IMP |  The International Mito Patients is a network of national patient organizations involved in mitochondrial disease. Mitochondrial disease is a rare disease with a limited number of patients per country. The national patient organizations which are a member of IMP each are active and powerful in their own countries. By joining forces IMP can represent a large group of patients and as such be their voice on an international level. The International Mito Patients is a network of national patient organizations involved in mitochondrial disease. Mitochondrial disease is a rare disease with a limited number of patients per country. The national patient organizations which are a member of IMP each are active and powerful in their own countries. By joining forces IMP can represent a large group of patients and as such be their voice on an international level. |
| International Society for Mountain Medicine | ISMM | The International Society for Mountain Medicine is an interdisciplinary society comprising about xx members worldwide. Its purpose is .. |
| International Society on Oxygen Transport to Tissue | ISOTT |
The International Society on Oxygen Transport to Tissue is an interdisciplinary society comprising about 250 members worldwide. Its purpose is to further the understanding of all aspects of the processes involved in the transport of oxygen from the air to its ultimate consumption in the cells of the various organs of the body. Founded in 1973, the society has been the leading platform for the presentation of many of the technological and conceptual developments within the field both at the meetings themselves and in the proceedings of the society. |
| International Standard Serial Number | ISSN | The International Standard Serial Number, ISSN, is a code used to identify periodical publications, independent of which media are used (print and/or electronic). - Bioenergetics Communications, BEC: ISSN 2791-4690 |
| International System of Units | SI | The International System of Units (SI) is the modern form of the metric system of units for use in all aspects of life, including international trade, manufacturing, security, health and safety, protection of the environment, and in the basic science that underpins all of these. The system of quantities underlying the SI and the equations relating them are based on the present description of nature and are familiar to all scientists, technologists and engineers. The definition of the SI units is established in terms of a set of seven defining constants. The complete system of units can be derived from the fixed values of these defining constants, expressed in the units of the SI. These seven defining constants are the most fundamental feature of the definition of the entire system of units. These particular constants were chosen after having been identified as being the best choice, taking into account the previous definition of the SI, which was based on seven base units, and progress in science (p. 125). |
| International Union of Pure and Applied Chemistry, IUPAC | IUPAC | The International Union of Pure and Applied Chemistry (IUPAC) celebrated in 2019 the 100th anniversary, which coincided with the International Year of the Periodic Table of Chemical Elements (IYPT 2019). IUPAC {Quote} notes that marking Mendeleev's achievement will show how the periodic table is central to connecting cultural, economic, and political dimensions of global society “through a common language” {end of Quote} (Horton 2019). 2019 is proclaimed as the International Year of the Periodic Table of Chemical Elements (IYPT 2019). For a common language in mitochondrial physiology and bioenergetics, the IUPAC Green book (Cohen et al 2008) is a most valuable resource, which unfortunately is largely neglected in bioenergetics textbooks. Integration of open systems and non-equilibrium thermodynamic approaches remains a challenge for developing a common language (Gnaiger 1993; BEC 2020.1). |
| International oxygraph course | IOC | International Oxygraph Course (IOC), see O2k-Workshops. |
| Internationale Gesellschaft fuer Regenerative Mitochondrien-Medizin | IGRMM e.V. | Organizer of |
| Intracellular oxygen | pO2,i | Physiological, intracellular oxygen pressure is significantly lower than air saturation under normoxia, hence respiratory measurements carried out at air saturation are effectively hyperoxic for cultured cells and isolated mitochondria. |
| Ionomycin | Imy | Ionomycin (Imy) is a ionophore used to raise intracellular [Ca2+]. |
| Isocitrate dehydrogenase | IDH | Isocitrate dehydrogenase forms 2-oxoglutarate from isocitrate in the TCA cycle. |
| Isolated mitochondria | imt | Isolated mitochondria, imt, are mitochondria separated from a tissue or cells by breaking the plasma membranes and attachments to the cytoskeleton, followed by centrifugation steps to separate the mitochondria from other components. |
| Japanese Society of Mitochondrial Research and Medicine | J-mit | The Japanese Society of Mitochondrial Research and Medicine (J-mit) was founded to share the latest knowledge on mitochondrial research. J-mit is the biggest Asian society of mitochondrial research and medicine and is a member of ASMRM. |
| Jmax | Jmax | Jmax is the maximum pathway flux (e.g. oxygen flux) obtained at saturating substrate concentration. Jmax is a function of metabolic state. In hyperbolic ADP or oxygen kinetics, Jmax is calculated by extrapolation of the hyperbolic function, with good agreement between the calculated and directly measured fluxes, when substrate levels are >20 times the c50 or p50. |
| Kelvin | K | The kelvin, symbol K, is the SI unit of thermodynamic temperature. It is defined by taking the fixed numerical value of the Boltzmann constant k to be 1.380 649 × 10−23 when expressed in the unit J x-1 K−1. |
| Kilogram | kg | The kilogram, symbol kg, is the SI unit of mass. It is defined by taking the fixed numerical value of the Planck constant h to be 6.626 070 15 × 10−34 when expressed in the unit J s, which is equal to kg m2 s−1, where the meter and the second are defined in terms of c and ΔνCs. |
| Korean Society of Mitochondrial Research and Medicine | KSMRM | The Korean Society of Mitochondrial Research and Medicine (KSMRM) is a member of ASMRM. |
| L/E coupling-control ratio | L/E | |
| L/P coupling-control ratio | L/P | |
| L/R coupling-control ratio | L/R | |
| LEAK respiration | L | |
| LEAK state with ATP | L(T) | |
| LEAK state with oligomycin | L(Omy) | |
| LEAK state without adenylates | L(n) | |
| Lactate dehydrogenase | LDH | Lactate dehydrogenase is a glycolytic marker enzyme in the cytosol, regenerating NAD+ from NADH and pyruvate, forming lactate. |
| Length | l [m] | Length l is an SI base quantity with SI base unit meter m. Quantities derived from length are area A [m2] and volume V [m3]. Length is an extensive quantity, increasing additively with the number of objects. The term 'height' h is used for length in cases of vertical position (see height of humans). Length of height per object, LUX [m·x-1] is length per unit-entity UX, in contrast to lentgth of a system, which may contain one or many entities, such as the length of a pipeline assembled from a number NX of individual pipes. Length is a quantity linked to direct sensory, practical experience, as reflected in terms related to length: long/short (height: tall/small). Terms such as 'long/short distance' are then used by analogy in the context of the more abstract quantity time (long/short duration). |
| Level flow | E | |
| Light-emitting diode | LED | A light-emitting diode (LED) is a light source (semiconductor), used in many every-day applications and specifically in fluorometry. LEDs are available for specific spectral ranges across wavelengths in the visible, ultraviolet, and infrared range. |
| Light-enhanced dark respiration | LEDR | Light-enhanced dark respiration LEDR is a sharp (negative) maximum of dark respiration in plants in response to illumination, measured immediately after switching off the light. LEDR is supported by respiratory substrates produced during photosynthesis and closely reflects light-enhanced photorespiration (Xue et al 1996). Based on this assumption, the total photosynthetic oxygen flux TP is calculated as the sum of the measured net photosynthetic oxygen flux NP plus the absolute value of LEDR. |
| Limiting oxygen pressure | pl | The limiting oxygen pressure, pl, is defined as the partial oxygen pressure, pO2, below which anaerobic catabolism is activated to contribute to total ATP generation. The limiting oxygen pressure, pl, may be substantially lower than the critical oxygen pressure, pc, below which aerobic catabolism (respiration or oxygen consumption) declines significantly. |
| Limiting pO2 | plim | In the transition from aerobic to anaerobic metabolism, there is a limiting pO2, plim, below which anaerobic energy flux is switched on and CR ratios become more exothermic than the oxycaloric equivalent. plim may be significanlty below the critical pO2. |
| Living Communications | LC | With Living Communications, Bioenergetics Communications (BEC) takes the next step from pre-print to re-print. The concept of Living Communications pursues a novel culture of scientific communication, addressing the conflict between long-term elaboration and validation of results versus sharing without delay improved methods and preliminary findings. Following the preprint concept, updates may be posted on the BEC website of the resource publication. Updated versions of Living Communications are submitted for Open Peer Review with full traceability. In contrast to static papers, evolution of Living Communications is more resourceful and efficient than a ‘new’ publication. Living Communications provide a pathway along the scientific culture of lively debate towards tested and trusted milestones of research, from pre-print to re-print, from initial steps to next steps. |
| Living cells | ce | Cell viability in living cells should be >95 % for various experimental investigations, including cell respirometry. Viable cells (vce) are characterized by an intact plasma membrane barrier function. The total cell count (Nce) is the sum of viable cells (Nvce) and dead cells (Ndce). In contrast, the plasma membrane can be permeabilized selectively by mild detergents (digitonin), to obtain the mt-preparation of permeabilized cells used for cell ergometry. Living cells are frequently labelled as intact cells in the sense of the total cell count, but intact may suggest dual meanings of viable or unaffected by a disease or mitochondrial injury. |
| MITOEAGLE in MitoGlobal | MitoEAGLE |
The objective of the MitoEAGLE network is to improve our knowledge on mitochondrial function in health and disease related to Evolution, Age, Gender, Lifestyle and Environment. |
| Magnesium Green | MgG | Magnesium Green (MgG) is an extrinsic fluorophore that fluoresces when bound to Mg2+ and is used for measuring mitochondrial ATP production by mitochondrial preparations. Determination of mitochondrial ATP production is based on the different dissociation constants of Mg2+ for ADP and ATP, and the exchange of one ATP for one ADP across the mitochondrial inner membrane by the adenine nucleotide translocase (ANT). Using the dissociation constants for ADP-Mg2+ and ATP-Mg2+ and initial concentrations of ADP, ATP and Mg2+, the change in ATP concentration in the medium is calculated, which reflects mitochondrial ATP production. |
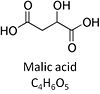
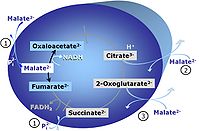
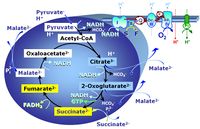
| Malate | M |
Malic acid, C4H6O5, occurs under physiological conditions as the anion malate2-, M, with pKa1 = 3.40 and pKa2 = 5.20. L-Malate is formed from fumarate in the TCA cycle in the mitochondrial matrix, where it is the substrate of malate dehydrogenase oxidized to oxaloacetate. Malate is also formed in the cytosol. It cannot permeate through the lipid bilayer of membranes and hence requires a carrier (dicarboxylate carrier, tricarboxylate carrier and 2-oxoglutarate carrier). Malate alone cannot support respiration of mt-preparations from most tissues, since oxaloacetate accumulates in the absence of pyruvate or glutamate. Malate is a type N substrate (N) required for the FAO-pathway. In the presence of anaplerotic pathways (e.g., mitochondrial malic enzyme, mtME) the capacity of the FAO-pathway can be overestimated due to a contribution of NADH-linked respiration, F(N) (see SUIT-002). |
| Malate dehydrogenase | mtMDH | Mitochondrial malate dehydrogenase is localized in the mitochondrial matrix and oxidizes malate, generated from fumarate by fumarase, to oxaloacetate, reducing NAD+ to NADH+H+ in the TCA cycle. Malate is added as a substrate in most N-pathway control states. |
| Malate-anaplerotic pathway control state | M | M: Malate alone does not support respiration of mt-preparations if oxaloacetate cannot be metabolized further in the absence of a source of acetyl-CoA. Transport of oxaloacetate across the inner mt-membrane is restricted particularly in liver. Mitochondrial citrate and 2-oxoglutarate (α-ketoglutarate) are depleted by antiport with malate. Succinate is lost from the mitochondria through the dicarboxylate carrier. OXPHOS capacity with malate alone is only 1.3% of that with Pyruvate&Malate in isolated rat skeletal muscle mitochondria. However, many mammalian and non-mammalian mitochondria have a mt-isoform of NADP+- or NAD(P)+-dependent malic enzyme (mtME), the latter being particularly active in proliferating cells. Then the anaplerotic pathway control state with malate alone (aN) supports high respiratory activities comparable to the NADH-linked pathway control states (N) with pyruvate&malate or glutamate&malate substrate combinations (PM-pathway control state, GM-pathway control state). |
| Malic enzyme | mtME | Malic enzyme (ME; EC 1.1.1.40) catalyzes the oxidative decarboxylation of L-malate to pyruvate with the concomitant reduction of the dinucleotide cofactor NAD+ or NADP+ and a requirement for divalent cations (Mg2+ or Mn2+) as cofactors. NAD(P)+ + L-malate2- <--> NAD(P)H + pyruvate- + CO2 Three groups of ME are distinguished (i) NAD+- and (ii) NADP+-dependent ME specific for NAD+ or NADP+, respectively, and (iii) NAD(P)+- dependent ME with dual specificity for NAD+ or NADP+ as cofactor. Three isoforms of ME have been identified in mammals: cytosolic NADP+-dependent ME (cNADP-ME or ME1), mitochondrial NAD(P)+-dependent ME (mtNAD-ME or ME2; with NAD+ or NADP+ as cofactor, preference for NAD+ under physiological conditions), and mitochondrial NADP+-dependent ME (mtNADP-ME or ME3). mtNAD-ME plays an important role in anaplerosis when glucose is limiting, particularly in heart and skeletal muscle. Tartronic acid (hydroxymalonic acid) is an inhibitor of ME. |
| Malonate | Mna | Malonate (malonic acid) is a competitive inhibitor of succinate dehydrogenase (Complex II). Malonate is a substrate of malonyl-CoA synthase. |
| Mark information | Marks | » See Marks - DatLab |
| Mark statistics - DatLab | F2 | In Mark statistics one Plot is selected as a source for Marks over sections of time. Values (e.g. medians) are displayed for these time sections of the source plot and of all selected plots. |
| Matrix-ETS | matrix-ETS | The component of the electron transfer system located in the mitochondrial matrix (matrix-ETS) is distringuished from the ETS bound to the mt-inner membrane (membrane-ETS). Electron transfer and corresponding OXPHOS capacities are classically studied in mitochondrial preparations as oxygen consumption supported by various fuel substrates undergoing partial oxidation in the mt-matrix, such as pyruvate, malate, succinate, and others. |
| Melatonin | aMT | Melatonin (N-acetyl-5-methoxytryptamine, aMT) is a highly conserved molecule present in unicellular to vertebrate organisms. Melatonin is synthesized from tryptophan in the pinealocytes by the pineal gland and also is produced in other organs, tissues and fluids (extrapineal melatonin). Melatonin has lipophilic and hydrophilic nature which allows it to cross biological membranes. Therefore, melatonin is present in all subcellular compartments predominantly in the nucleus and mitochondria. Melatonin has pleiotropic functions with powerful antioxidant, anti-inflammatory and oncostatic effects with a wide spectrum of action particularly at the level of mitochondria. » MiPNet article |
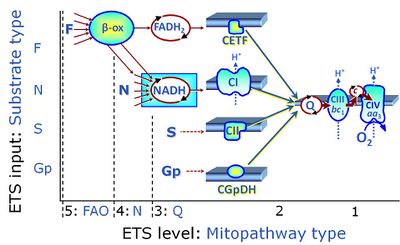
| Membrane-bound ET pathway | mET-pathway | The membrane-bound electron transfer pathway (mET pathway) consists in mitochondria mainly of respiratory complexes CI, CII, electron transferring flavoprotein complex (CETF), glycerophosphate dehydrogenase complex (CGpDH), and choline dehydrogenase, with convergent electron flow at the Q-junction (Coenzyme Q), and the two downstream respiratory complexes connected by cytochrome c, CIII and CIV, with oxygen as the final electron acceptor. The mET-pathway is the terminal (downstream) module of the mitochondrial ET pathway and can be isolated from the ET-pathway in submitochondrial particles (SmtP). |
| Metabolic control variable | X | A metabolic control variable X causes the transition between a background state Y (background rate YX) and a reference state Z (reference rate ZX). X may be a stimulator or activator of flux, inducing the step change from background to reference steady state (Y to Z). Alternatively, X may be an inhibitor of flux, absent in the reference state but present in the background state (step change from Z to Y). |
| Meter | m | The meter, symbol m, is the SI unit of the SI base quantity length l. It is defined by taking the fixed numerical value of the speed of light c in vacuum to be 299 792 458 when expressed in the unit m·s−1, where the second is defined in terms of the caesium frequency ΔνCs. |
| Methylmalonic acid | Mma | Methylmalonic acid (Mma) is a common intermediate in many catabolic processes. In methylmalonic acidemia mitochondrial dysfunction can be observed, related to accumulation of Mma and associated with neurological symptoms. |
| MiP-Collection | MiP-Collection | Mitochondrial Physiology - Historical Collection Aims The growing MiP-Collection aims at preserving scientific instruments that are of historical importance in the field of bioenergetics and mitochondrial physiology. The fast turnover of scientific equipment makes obsolete even comparatively recent instrumentation. The Oroboros O2k was the first commercial mitochondrial respirometer using a computer for data acquisition. Today, chart recorders are nearly forgotten. Due to limitations of storage space, unused scientific equipment is disposed of, despite its potential historical value. The disposal of some unique apparatus constitutes an irreversible loss to science and society, and to the continued appreciation of the foundations of our scientific discipline. You may consider to make items of scientific historical interest in mitochondrial physiology available to the MiP-Collection. These items of the MiP-Collection may specifically include historically valuable
|
| MiP03 | MiP03 | Mitochondrial Preservation Medium, MiP03, developed for preservation of isolated mitochondria. |
| MiPMap | MiPMap |
The project Mitochondrial Physiology Map (MiPMap) is initiated to provide an overview of mitochondrial properties in cell types, tissues and species. As part of Bioblast, MiPMap may be considered as an information synthase for Comparative Mitochondrial Physiology. Establishing a comprehensive database will require global input and cooperation. A comparative database of mitochondrial physiology may provide the key for understanding the functional implications of mitochondrial diversity from mouse to man, and evaluation of altered mitochondrial respiratory control patterns in health and disease (Gnaiger 2009). |
| MiPNet-Publication | MiPNet | MiPNet is the abbreviation for the OROBOROS Journal Mitochondrial Physiology Network, including chapters of the O2k-Manual, O2k-Procedures, O2k-Workshops, and other announcements, starting with MiPNet 01 in 1996. See also »MiPNet. |
| MiPSociety | MiP |
The Mitochondrial Physiology Society (MiP) has been founded to organize MiPconferences, MiPschools, and MiPworkshops worldwide. MiP has been founded at the Third Conference on Mitochondrial Physiology (MiP2003, Schroecken, Austria). The MiPsociety is an international organization, based in Europe and operating world-wide. |
| MiR05 | MiR05 | Mitochondrial respiration medium, MiR05, developed for oxygraph incubations of mitochondrial preparations. Respiration of living cells may be assessed in MiR05 by adding pyruvate (P) as an external source. MiR06 = MiR05 + catalase. MiR05Cr = MiR05 + creatine. |
| MiR05Cr | MiR05Cr | Mitochondrial respiration medium, MiR05Cr, developed for oxygraph incubations of mitochondrial preparations - permeabilized muscle fibers. |
| MiR06 | MiR06 | Mitochondrial respiration medium, MiR06, developed for oxygraph incubations of mitochondrial preparations. MiR06 = MiR05 plus catalase. MiR06Cr = MiR06 plus creatine. |
| MiR06Cr | MiR06Cr | Mitochondrial respiration medium, MiR06Cr, developed for oxygraph incubations of mitochondrial preparations - permeabilized muscle fibers. |
| MiRK03 | MiRK03 | Mitochondrial respiration medium, MiRK03, modified after a medium described by Komary 2010 Biochim Biophys Acta, intended for use as medium for H2O2 production measurement with Amplex Red. |
| Microxia | microx | Microxia (deep hypoxia) is obtained when trace amounts of O2 exert a stimulatory effect on respiration above the level where metabolism is switched to a purely anaerobic mode. |
| MitoAction | MitoAction |  The mission of MitoAction is to improve quality of life for all who are affected by mitochondrial disorders through support, education and advocacy initiatives. The mission of MitoAction is to improve quality of life for all who are affected by mitochondrial disorders through support, education and advocacy initiatives. |
| MitoCanada Foundation | mitoCanada | The MitoCanada Foundation. The MitoCanada Foundation is Canada’s only not-for-profit organization focused on mitochondrial disease. Since its founding in 2010, MitoCanada has dedicated over $1 million to fund the work of leading Canadian scientists and to support national awareness and support programs. The MitoCanada Foundation is committed to ensuring that those who live with mitochondrial disease are able to enjoy the best possible quality of life until there is a cure. |
| MitoFit DOI Data Center | MitoFit DOI DC | The MitoFit DOI Data Center is responsible for the provision of digital identifiers, for the storage and ensuring the persistence of the scientific objects, the provision of access, review process and maintenance of the Metadata, and quality control. |
| MitoFit Preprints | MitoFit Prep | MitoFit Preprints is an Open Access preprint server for mitochondrial physiology and bioenergetics. |
| MitoFit registered project | MitoFit-RP | MitoFit registered projects are announced with reference to MitoFit protocols as publicly deposited protocols. Project registration is a two-phase process. Guidelines will be defined. (1) Pre-registration of a project requires submission to a MitoFit moderator (editor), including protocol details with reference to MitoPedia protocols, or with submission of protocols for publication (Open Access) in MitoPedia. The MitoFit (Bioblast) editors will edit the submitted protocols (layout) and insert into Bioblast submitted pre-registrations and protocols. (2) MitoFit moderators (editors) will set up a MitoFit accreditation panel, in which the registrant will be included (perhaps not in the long run, to avoid conflict of interests) and/or for which the registrant can suggest delegates (compare peer review). Accredited MitoFit protocols are labelled as MitoFit accredited, and the pre-registered MitoFit project becomes labelled and listed as MitoFit registered project (MitoFit accredited). This is possible before (advance registration), during progress, and after completion of a study (post-registration). A MitoFit registered project receives a code for feeding data into the MitoFit data repository. |
| MitoKit-CII/Malonate-nv | Mnanv | MitoKit-CII/Malonate-nv (diacetoxymethyl malonate) is a plasma membrane-permeable prodrug (permeable malonate; Mnanv) that diffuses across the plasma membrane. Cleavage of diacetoxymethyl groups is mediated by intracellular esterases, thus releasing malonate in the intracellular space. Abliva #: 01-161-s2 |
| MitoKit-CII/Succinate-nv | Snv | MitoKit-CII/Succinate-nv (diacetoxymethyl succinate) is a plasma membrane-permeable prodrug (permeable succinate; Snv) that diffuses across the plasma membrane. Cleavage of diacetoxymethyl groups is mediated by intracellular esterases, thus releasing succinate in the intracellular space. Abliva #: 01-118-s4 |
| MitoOx1 | MitoOx1 | Mitochondrial respiration medium, MitoOx1, used by the Budapest groups for respirometry und Amplex Red trials. |
| MitoOx2 | MitoOx2 | Mitochondrial respiration medium, MitoOx2, developed for oxygraph incubations of mitochondrial preparations to measure the H2O2 production. MitoOx2 yields a higher optical sensitivity and lower "drift" (oxidation of the fluorophore precurcor without H2O2 present) for Amplex UltraRed(R) than e.g. MiR05. |
| Mitochondria | mt | Mitochondria (Greek mitos: thread; chondros: granule) are small structures within cells, which function in cell respiration as powerhouses or batteries. Mitochondria belong to the bioblasts of Richard Altmann. Abbreviation: mt, as generally used in mtDNA. Singular: mitochondrion (bioblast); plural: mitochondria (bioblasts). |
| Mitochondria Interest Group | MIG |
The Mitochondria Interest Group (MIG) is an Inter-Institute Interest Group at the National Institutes of Health (NIH), with members worldwide! MIG is concerned with all aspects of the mitochondrion and diseases in which the mitochondrion is involved. We hold monthly meetings, usually on the second Monday of the month (except when it is a Federal holiday or other special exceptions). [email protected] is an Email list moderated by Ph.D. Steven Zullo as an interactive information platform, with free subscritpion to this mitochondrial network. List members are reminded of their responsibility to critically evaluate the content of the postings. The information, opinions, data, and statements contained herein are not necessarily those of the U. S. Government, the National Institutes of Health (NIH), or MIG and should not be interpreted, acted on or represented as such. |
| Mitochondria Research Society | MRS |
The Mitochondria Research Society (MRS) is a nonprofit international organization of scientists and physicians. The purpose of MRS is to find a cure for mitochondrial diseases by promoting research on basic science of mitochondria, mitochondrial pathogenesis, prevention, diagnosis and treatment through out the world. |
| Mitochondria-Targeted Drug Development | hansonwade |
The Mitochondria-Targeted Drug Development Summit was first established in 2021, as an online conference. Due to its success and unmatched focus, the 2nd edition returns to Boston this March 2022. This is the only industry-led meeting that unites key stakeholders under a mutual and ambitious objective of accelerating the discovery and development of novel drugs that target mitochondrial functions for chronic, primary mitochondrial diseases, muscular dystrophy, metabolic disorders, and neurodegenerative diseases. Join our speakers from GenSight Biologics, Abliva, Reneo Pharma, Mito BioPharma, Mitokinin and more with exciting networking opportunities, panel discussions and dedicated roundtables. |
| Mitochondrial ATP-sensitive K+ channel | mtKATP | The mitochondrial ATP-sensitive K+ channel (mtKATP or mitoKATP). |
| Mitochondrial European Education Training | MEET | The Mitochondrial European Education Training (MEET) MEET is a project started on January 2013. MEET network is composed by a multi-partner project that intends to mobilize the critical mass of expertise, by linking partners from 8 different countries, among which 8 world-leading basic science and clinical centers of excellence, an 1 SME with direct interest in mitochondrial medicine and 3 associated partners that provide for all trainees no-scientific training. MEET is training 11 ESRs and 3 ERs coming from all over the world supervised in their research by 15 mentors and by their collaborators. MEET combine the efforts of leading clinicians with those of more basic oriented groups and will have important implications for the comprehension and treatment of mitochondria-related pathologies. |
| Mitochondrial Medicine Society | MMS |
The Mitochondrial Medicine Society (MMS) was founded in 2000 and represents an international group of physicians, researchers and clinicians working towards the better diagnosis, management, and treatment of mitochondrial diseases. |
| Mitochondrial Physiology Network | Mitochondr physiol network, MiPNet | The Mitochondrial Physiology Network is the on-line Oroboros journal. |
| Mitochondrial competence | mt-competence; MitoCom | Mitochondrial metabolic competence is the organelle's capacity to provide adequate amounts of ATP in due time, by adjusting the mt-membrane potential, mt-redox states and the ATP/ADP ratio according to the metabolic requirements of the cell. The term mitochondrial competence is also known in a genetic context: Mammalian mitochondria possess a natural competence for DNA import. MitoCom_O2k-Fluorometer is a Mitochondrial Competence network, the nucleus of which is formed by the K-Regio project MitoCom Tyrol. |
| Mitochondrial concentration | CmtE | Mitochondrial concentration is CmtE = mtE·V-1 [mtEU·m-3]. mt-Concentration is an experimental variable, dependent on sample concentration. |
| Mitochondrial content | mtENX | Mitochondrial content per object X is mtENX = mtE·NX-1 [mtEU·x-1]. |
| Mitochondrial density | DmtE | Specific mitochondrial density is DmtE = mtE·mX-1 [mtEU·kg-1]. If the amount of mitochondria, mtE, is expressed as mitochondrial mass, then DmtE is the mass fraction of mitochondria in the sample. If mtE is expressed as mitochondrial volume, Vmt, and the mass of sample, mX, is replaced by volume of sample, VX, then DmtE is the volume fraction of mitochondria in the sample. |
| Mitochondrial free radical theory of aging | MFRTA | The mitochondrial free radical theory of aging goes back to Harman (1956) and ranks among the most popular theories of aging. It is based on postulates which are not unequivocally supported by observation (Bratic, Larsson 2013): (i) Mitochondrial ROS production increases with age caused by progressive mitochondrial dysfunction; (ii) antioxidat capacity declines with age; (iii) mutations of somatic mtDNA accumulate during aging; (iv) a vicious cycle occurs of increased ROS production caused by mtDNA mutations and degenerated mt-function, and due to ROS-induced ROS production. |
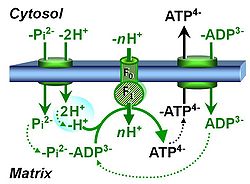
| Mitochondrial inner membrane | mtIM | The mitochondrial inner membrane mtIM is the structure harboring the membrane-bound electron transfer system ETS including the respiratory complexes working as hydrogen ion pumps, the mt-phosphorylation system including the hydrogen ion pump ATP synthase, several substrate transporters involved in the electron transfer pathway, and a variety of other ion pumps that carry proton charge (Ca2+, Mg2+). The protonmotive force is the electrochemical potential difference across the mtIM generated by the hydrogen ion pumps of the . |
| Mitochondrial marker | mt-marker | Mitochondrial markers are structural or functional properties that are specific for mitochondria. A structural mt-marker is the area of the inner mt-membrane or mt-volume determined stereologically, which has its limitations due to different states of swelling. If mt-area is determined by electron microscopy, the statistical challenge has to be met to convert area into a volume. When fluorescent dyes are used as mt-marker, distinction is necessary between mt-membrane potential dependent and independent dyes. mtDNA or cardiolipin content may be considered as a mt-marker. Mitochondrial marker enzymes may be determined as molecular (amount of protein) or functional properties (enzyme activities). Respiratory capacity in a defined respiratory state of a mt-preparation can be considered as a functional mt-marker, in which case respiration in other respiratory states is expressed as flux control ratios. » MiPNet article |
| Mitochondrial matrix | mt-matrix | The mitochondrial matrix (mt-matrix) is enclosed by the mt-inner membrane mtIM. The terms mitochondrial matrix space or mitochondrial lumen are used synonymously. The mt-matrix contains the enzymes of the tricarboxylic acid cycle, fatty acid oxidation and a variety of enzymes that have cytosolic counterparts (e.g. glutamate dehydrogenase, malic enzyme). Metabolite concentrations, such as the concentrations of fuel substrates, adenylates (ATP, ADP, AMP) and redox systems (NADH), can be very different in the mt-matrix, the mt-intermembrane space, and the cytosol. The finestructure of the gel-like mt-matrix is subject of current research. |
| Mitochondrial membrane potential | mtMP, ΔΨp+, ΔelFep+ [V] | The mitochondrial membrane potential difference, mtMP or ΔΨp+ = ΔelFep+, is the electric part of the protonmotive force, Δp = ΔmFeH+.
ΔΨp+ is the potential difference across the mitochondrial inner membrane (mtIM), expressed in the electric unit of volt [V]. Electric force of the mitochondrial membrane potential is the electric energy change per ‘motive’ charge or per charge moved across the transmembrane potential difference, with the number of ‘motive’ charges expressed in the unit coulomb [C]. |
| Mitochondrial outer membrane | mtOM | The mitochondrial outer membrane is the incapsulating membrane which is osmotically not active and contains the cytochrome b5 enzyme similar to that found in the endoplasmatic reticulum, the translocases of the outer membrane, monoaminooxidase, the palmitoyl-CoA synthetase and carnytil-CoA transferase 1. |
| Mitochondrial preparations | mtprep | Mitochondrial preparations (mtprep) are isolated mitochondria (imt), tissue homogenate (thom), mechanically or chemically permeabilized tissue (permeabilized fibers, pfi) or permeabilized cells (pce). In mtprep the plasma membranes are either removed (imt) or mechanically (thom) and chemically permeabilized (pfi), while mitochondrial functional integrity and to a large extent mt-structure are maintained in incubation media optimized to support mitochondrial physiological performance. According to this definition, submitochondrial particles (smtp) are not a mtprep, since mitochondrial structure is altered although specific mitochondrial functions are preserved. |
| Mitochondrial respiration media: comparison | MiR | Mitochondrial respiratory capacity and control are compared in different mitochondrial respiration media, MiRs, to evaluate the quality of MiRs in preserving mitochondrial function and to harmonize results obtained in various studies using different MiRs. In some cases alterations of the formulation are incorporated to optimize conditions for the simultaneous measurement of multiple parameters, e.g. respiration and ROS production. |
| Mitochondrial states and rates - terminology beyond MitoEAGLE 2020 | States and rates | 666 coauthors of the 'MitoEAGLE white paper' [1] collaborated to reach a consensus on terminology related to mitochondrial respiratory states and rates. This page is intended to prepare a questionnaire and follow-up publication. |
| Mitochondrial transcription factor A | TFAM | The transcription factor A is a gene that encodes a mitochondrial transcription factor that is a key activator of mitochondrial transcription as well as a participant in mitochondrial genome replication. TFAM is downstream of PGC-1alpha. |
| Molar mass | M [kg·mol-1]; [g·mol-1] | Molar mass M is the mass of a chemical compound divided by its amount-of-substance measured in moles. It is defined as MB = m/nB, where m is the total mass of a sample of pure substance and nB is the amount of substance B given in moles. The definition applies to pure substance. The molar mass allows for converting between the mass of a substance and its amount for bulk quantities. It is calculated as the sum of standard atomic weights of all atoms that form one entity of the substance. The appropriate SI base units is kg·mol-1. However, for historical as well as usability reasons, g·mol-1 is almost always used instead. |
| Mole | mol | The mole [mol] is the SI base unit for the amount of substance of a system that contains 6.02214076·1023 specified elementary entities (see Avogadro constant). The elementary entities must be specified and may be atoms, molecules, ions, electrons, other particles, or specified groups of such particles. |
| Monoamine oxidase | MAO | Monoamine oxidases are enzymes bound to the outer membrane of mitochondria and they catalyze the oxidative deamination of monoamines. Oxygen is used to remove an amine group from a molecule, resulting in the corresponding aldehyde and ammonia. Monoamine oxidases contain the covalently bound cofactor FAD and are, thus, classified as flavoproteins. |
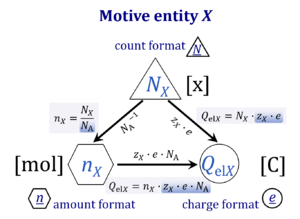
| Motive entity | Xtr [MU] | . A motive entity Xtr is an entity involved in a transformation including spacial transfer. Motive entities (transformants) are expressed in different motive units [MU] depending on the energy transformation under study and the chosen format. Flows are defined as advancement in terms of stoichiometric motive entities per time. Isomorphic forces are partial derivatives of Gibbs energy per advancement. Ions carrying a positive charge (cations) or negative charge (anions) may be considered as a paradigm of motive entities, since Faraday did not coin but introduced the term 'ion', which is old Greek for 'going' — advancing to the cathode or anode and thus generating an electric current. |
| Motive unit | MU | The motive unit [MU] is the variable SI unit in which the motive entity (transformant) of a transformation is expressed, which depends on the energy transformation under study and on the chosen format. Fundamental MU for electrochemical transformations are:
For the protonmotive force the motive entity is the proton with charge number z=1. The protonmotive force is expressed in the electrical or molar format with MU J/C=V or J/mol=Jol, respectively. The conjugated flows, I, are expressed in corresponding electrical or molar formats, C/s = A or mol/s, respectively. The charge number, z, has to be considered in the conversion of motive units (compare Table below), if a change not only of units but a transition between the entity elementary charge and an entity with charge number different from unity is involved (e.g., O2 with z=4 in a redox reaction). The ratio of elementary charges per reacting O2 molecule (zO2=4) is multiplied by the elementary charge (e, coulombs per proton), which yields coulombs per O2 [C∙x-1]. This in turn is multiplied with the Avogadro constant, NA (O2 molecules per mole O2 [x∙mol-1]), thus obtaining for zeNA the ratio of elementary charges [C] per amount of O2 [mol-1]. The conversion factor for O2 is 385.94132 C∙mmol-1. |
| Mouse control: Mark | Ctrl+M | The mark mode is active by default, can be selected in the menu or by [Ctrl+M]. If Mouse control: Mark is enabled, specific sections of the experiment can be marked in each plot. Usually, marks are set on the plot for oxygen concentration for calibration, whereas marks on the plot for oxygen flux are set for exporting the median or average of flux to a table. »More details: Marks - DatLab. |
| Mouse control: Zoom | Ctrl+Z | Select Mouse Control: Zoom in the Graph-menu or press [Ctrl+Z]. |
| MtOM | mtOM | The mitochondrial outer membrane |
| Myxothiazol | Myx | Myxothiazol Myx is an inhibitor of Complex III (CIII). CIII also inhibits CI. Myxothiazol binds to the Qo site of CIII (close to cytochrome bL) and inhibits the transfer of electrons from reduced QH2 to the Rieske iron sulfur protein. |
| N-ethylmaleimide | Nem | N-ethylmaleimide is an organic compound that is derived from maleic acid and blocks endogenous Pi transport. |
| N/NS pathway control ratio | N/NS | The N/NS pathway control ratio is obtained when succinate is added to N-linked respiration in a defined coupling state. N and NS are abbreviations for respiration in the N-pathway control state (with pyruvate, glutamate, malate, or other ETS competent N-linked substrate combinations) and the NS-pathway control state (N in combination with succinate). NS indicates respiration with a cocktail of substrates supporting the N- and S-pathways. |
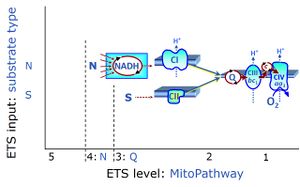
| N/S pathway control ratio | N/S | The N/S pathway control ratio is obtained from SUIT protocols when the N-pathway flux and S-pathway flux are measured in the same coupling control state. The N/S pathway control ratio may be larger or smaller than 1.0, depending on the mitochondrial source and various mitochondrial injuries. The S-pathway control state may be selected preferentially as reference state, if mitochondria are studied with respect to N-pathway injuries. |
| NADH | NADH | NAD+ and NADH: see Nicotinamide adenine dinucleotide. |
| NADH electron transfer-pathway state | N |
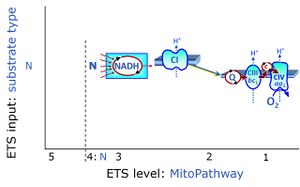
The NADH electron transfer-pathway state (N) is obtained by addition of NADH-linked substrates (CI-linked), feeding electrons into the N-junction catalyzed by various mt-dehydrogenases. N-supported flux is induced in mt-preparations by the addition of NADH-generating substrate combinations of pyruvate (P), glutamate (G), malate (M), oxaloacetate (Oa), oxoglutarate (Og), citrate, hydroxybutyrate. These N-junction substrates are (indirectly) linked to Complex I by the corresponding dehydrogenase-catalyzed reactions reducing NAD+ to NADH+H+ + H+. The most commonly applied N-junction substrate combinations are: PM, GM, PGM. The malate-anaplerotic pathway control state (M alone) is a special case related to malic enzyme (mtME). The glutamate-anaplerotic pathway control state (G alone) supports respiration through glutamate dehydrogenase (mtGDH). Oxidation of tetrahydrofolate is a NAD(P)H linked pathway with formation of formate. In mt-preparations, succinate dehydrogenase (SDH; CII) is largely substrate-limited in N-linked respiration, due to metabolite depletion into the incubation medium. The residual involvement of S-linked respiration in the N-pathway control state can be further suppressed by the CII-inhibitor malonic acid). In the N-pathway control state ET pathway level 4 is active. |
| NS e-input | NS, CI&II | NS e-input or the NS-pathway control state is electron input from a combination of substrates for the N-pathway control state and S-pathway control state through Complexes CI and CII simultaneously into the Q-junction. NS e-input corresponds to TCA cycle function in vivo, with convergent electron flow through the Electron transfer pathway. In mt-preparations, NS e-input requires addition not only of NADH- (N-) linked substrates (pyruvate&malate or glutamate&malate), but of succinate (S) simultaneously, since metabolite depletion in the absence of succinate prevents a significant stimulation of S-linked respiration. For more details, see: Additive effect of convergent electron flow. |
| NS-N pathway control efficiency | jNS-N; jCI&II-CI | The NS-N pathway control efficiency, jNS-N = 1-N/NS, expresses the fractional change of flux when succinate is added to the N-pathway control state in a defined coupling-control state. |
| NS-S pathway control efficiency | jNS-S | The NS-S pathway control efficiency expresses the relative stimulation of succinate supported respiration (S) by NADH-linked substrates (N), with the S-pathway control state as the background state and the NS-pathway control state as the reference state. In typical SUIT protocols with type N and S substrates, flux in the NS-pathway control state NS is inhibited by rotenone to measure flux in the S-pathway control state, S(Rot) or S. Then the NS-S pathway control efficiency in the ET-coupling state is j(NS-S)E = (NSE-SE)/NSE The NS-S pathway control efficiency expresses the fractional change of flux in a defined coupling-control state when inhibition by rotenone is removed from flux under S-pathway control in the presence of a type N substrate combination. Experimentally rotenone Rot is added to the NS-state. The reversed protocol, adding N-substrates to a S-pathway control background does not provide a valid estimation of S-respiration with succinate in the absence of Rot, since oxaloacetate accumulates as a potent inhibitor of succinate dehydrogenase CII. |
| NS-pathway control state | NS, CI&II |
NS-pathway control is exerted in the NS-linked substrate state (flux in the NS-linked substrate state, NS; or Complex I&II, CI&II-linked substrate state). NS-OXPHOS capacity provides an estimate of physiologically relevant maximum mitochondrial respiratory capacity. NS is induced in mt-preparations by addition of NADH-generating substrates (N-pathway control state in combination with succinate (Succinate pathway; S). Whereas NS expresses substrate control in terms of substrate types (N and S), CI&II defines the same concept in terms of convergent electron transfer to the Q-junction (pathway control). NS is the abbreviation for the combination of NADH-linked substrates (N) and succinate (S). This physiological substrate combination is required for partial reconstitution of TCA cycle function and convergent electron-input into the Q-junction, to compensate for metabolite depletion into the incubation medium. NS in combination exerts an additive effect of convergent electron flow in most types of mitochondria. |
| NSGp | NSGp |
MitoPathway control state: NSGp
SUIT protocol: SUIT-038 This substrate combination supports convergent electron flow to the Q-junction. |
| Natoms O | natoms O | 0.5 nmol O2; in bioenergetics a variety of expressions is used for units of amount of half a nmol molecular oxygen (natoms oxygen; natoms O; ng.atom O; nmol O), with the identical meaning: 0.5 nmol O2. |
| Net P/E control ratio | (P-L)/E | |
| Net R/E control ratio | (R-L)/E | |
| Neurocon | Neurocon |
Neurocon is an Indian society organizing international conferences on neurodegenerative and neurodevelopmental diseases. |
| Nicotinamide adenine dinucleotide | NADH | Nicotinamide adenine dinucleotide, NAD+ and NADH (pyridine nucleotide coenzymes, NAD and NADP), is an oxidation-reduction coenzyme (redox cofactor; compare FADH2). In the NADH electron transfer-pathway state fuelled by type N-substrates, mt-matrix dehydrogenases generate NADH, the substrate of Complex I (CI). The reduced N-substrate RH2 is oxidized and NAD+ is reduced to NADH,:::: RH2 + NAD+ → NADH + H+ + RThe mt-NADH pool integrates the activity of the TCA cycle and various matrix dehydrogenases upstream of CI, and thus forms a junction or funnel of electron transfer to CI, the N-junction (compare F-junction, Q-junction). NAD+ and NADH are not permeable through the mt-inner membrane, mtIM. Therefore, an increase of mitochondrial respiration after the addition of NADH may indicate an alteration of the mtIM integrity. Cytosolic NADH is effectively made available for mitochondrial respiration through the malate-aspartate shuttle or glycerophosphate dehydrogenase Complex. |
| Nitric oxide synthase | NOS | Nitric oxide synthase, NOS, catalyzes the production of nitric oxide (NO•), which is a reactive nitrogen species. There are four types of NOS: neuronal NOS (nNOS), endothelial NOS (eNOS), inducible NOS (iNOS) and mitochondrial NOS (mtNOS). |
| Noncoupled respiration | E |
Noncoupled respiration is maximum electron flow in an open-transmembrane proton circuit mode of operation (see ET capacity). » MiPNet article |
| Normoxia | normox |
Normoxia is a reference state, frequently considered as air-level oxygen pressure at sea level (c. 20 kPa in water vapor saturated air) as environmental normoxia. Intracellular tissue normoxia is variable between organisms and tissues, and intracellular oxygen pressure is frequently well below air-level pO2 as a result of cellular (mainly mitochondrial) oxygen consumption and oxygen gradients along the respiratory cascade. Oxygen pressure drops from ambient normoxia of 20 kPa to alveolar normoxia of 13 kPa, while extracellular normoxia may be as low as 1 to 5 kPa in solid organs such as heart, brain, kidney and liver. Pericellular pO2 of cells growing in monolayer cell cultures may be hypoxic compared to tissue normoxia when grown in ambient normoxia (95 % air and 5 % CO2) and a high layer of culture medium causing oxygen diffusion limitation at high respiratory activity, but pericellular pO2 may be effectively hyperoxic in cells with low respiratory rate with a thin layer of culture medium (<2 mm). Intracellular oxygen levels in well-stirred suspended small cells (5 - 7 mm diameter; endothelial cells, fibroblasts) are close to ambient pO2 of the incubation medium, such that matching the experimental intracellular pO2 to the level of intracellular tissue normoxia requires lowering the ambient pO2 of the medium to avoid hyperoxia. |
| Nuclear receptors | NRs | Nuclear receptors are ligand-dependent transcription factors. |
| Nuclear respiratory factor 1 | NRF-1 | Nuclear respiratory factor 1 is a transcription factor downstream of PGC-1alpha involved in coordinated expression of nDNA and mtDNA. |
| Number | N | A number N is a count NX [x] divided by the elementary entity UX [x]. X must represent the same entity in both occurences. The elementary unit [x] cancels in the division by simplification, such that numbers (for example, numbers 8 or 24) are abstracted from the counted entity X. The concept of number is tightly entangled with units, counts and entities. |
| O2k | O2k | O2k - Oroboros O2k: the modular system for high-resolution respirometry. |
| O2k control (active) | F7 | DatLab 8: Change settings of the connected O2k and current measurement. DatLab 7 : to modify instrumental settings: O2k control; to modify settings of specific channels: O2k configuration. |
| O2k control - DatLab 7 | F7 | After selection of an O2k setup in the O2k control [F7] window, followed by a left-click Send to O2k, only the following control functions are routinely required during experimental operations. |
| O2k info (view) | Ctrl+F7 | O2k info (view) displays the experimental settings from the selected file, but does not allow modifying them. The only exception is the O2 sensor number, that can be modified by clicking on the edit button below the field. If the sensor number is modified after the recording, a warning appears next to the field, for quality control, informing that it has been changed. If more previously recorded files are open while running a measurement, O2k control (active) will always show the settings of the active recording, while O2k info (view) will show the settings of the selected file. The O2k info (view) option is disabled when the current recording file is selected. |
| O2k-Network Reference Laboratory | O2k-Network Lab |
O2k-Network Reference Laboratories build a WorldWide network on high-resolution respirometry and mitochondrial physiology, the Oroboros O2k-Network. |
| O2k-sV-Module | O2k-sV-Module |
The O2k-sV-Module is the O2k small-volume module, comprised of two Duran® glass chambers of 12 mm inner diameter specifically developed to perform high-resolution respirometry with reduced amounts of biological sample, and all the components necessary for a smaller operation volume V of 0.5 mL. The current DatLab version is included in the delivery of this revolutionary module. |
| OXPHOS capacity | P | |
| OctGM | OctGM | OctGM: Octanoylcarnitine & Glutamate & Malate. MitoPathway control state: FN |
| OctGMS | OctGMS | OctGMS: Octanoylcarnitine &Glutamate & Malate& Succinate. MitoPathway control state: FNS |
| OctM pathway control state | OctM | OctM: Octanoylcarnitine & Malate. MitoPathway control state: F SUIT protocols: SUIT-002, SUIT-015, SUIT-016, SUIT-017 Respiratory stimulation of the FAO-pathway, F, by fatty acid FA in the presence of malate M. Malate is a type N substrate (N), required for the F-pathway. In the presence of anaplerotic pathways (e.g., mitochondrial malic enzyme, mtME) the F-pathway capacity is overestimated, if there is an added contribution of NADH-linked respiration, F(N) (see SUIT-002). The FA concentration has to be optimized to saturate the FAO-pathway, without inhibiting or uncoupling respiration. Low concentration of malate, typically 0.1 mM, does not saturate the N-pathway; but saturates the F-pathway. High concentration of malate, typically 2 mM, saturates the N-pathway. |
| OctPGM pathway control state | OctPGM | OctPGM: Octanoylcarnitine & Pyruvate & Glutamate & Malate. MitoPathway control state: FN SUIT protocols: SUIT-002
|
| OctPGMS pathway control state | OctPGMS | OctPGMS: Octanoylcarnitine & Pyruvate & Glutamate & Malate & Succinate. MitoPathway control state: FNS SUIT protocol: SUIT-001, SUIT-002, SUIT-015 This substrate combination supports convergent electron flow to the Q-junction. |
| OctPGMSGp pathway control state | OctPGMSGp | OctPGMSGp: Octanoylcarnitine & Pyruvate & Glutamate & Malate & Succinate & Glycerophosphate. MitoPathway control state: FNSGp SUIT protocol: SUIT-002 This substrate combination supports convergent electron flow to the Q-junction. |
| OctPM pathway control state | OctPM | OctPM: Octanoylcarnitine & Pyruvate & Malate. MitoPathway control state: FN SUIT protocol: SUIT-002, SUIT-005 This substrate combination supports N-linked flux which is typically higher than FAO capacity (F/FN<0 in the OXPHOS state). In SUIT-RP1, PMOct is induced after PM(E), to evaluate any additive effect of adding Oct. In SUIT-RP2, FAO OXPHOS capacity is measured first, testing for the effect of increasing malate concentration (compare malate-anaplerotic pathway control state, M alone), and pyruvate is added to compare FAO as the background state with FN as the reference state. |
| OctPMS | OctPMS | OctPMS: Octanoylcarnitine & Pyruvate & Malate & Succinate. MitoPathway control state: FNS SUIT protocol: SUIT-005 |
| Octanoate | Oca | Octanoate (octanoic acid). C8H16O2 Common name: Caprylic acid. |
| Octanoylcarnitine | Oct | Octanoylcarnitine is a medium-chain fatty acid (octanoic acid: eight-carbon saturated fatty acid) covalently linked to carnitine, frequently applied as a substrate for fatty acid oxidation (FAO) in mitochondrial preparations. |
| Oligomycin | Omy | Oligomycin (Omy) is an inhibitor of ATP synthase by blocking its proton channel (Fo subunit), which is necessary for oxidative phosphorylation of ADP to ATP (energy production). The inhibition of ATP synthesis also inhibits respiration. In OXPHOS analysis, Omy is used to induce a LEAK respiration state of respiration (abbreviated as L(Omy) to differentiate from L(n), LEAK state in the absence of ADP). |
| Open - DatLab | Ctrl+O | Open a previously recorded DatLab file. |
| Open Access | OA | Open Access (OA) academic articles comprise all different forms of published research that are distributed online, free of charge and with an open license to facilitate the distribution and reuse. The open access repositories serve as the perfect vehicle to transmit free knowledge, including but not limited to peer-reviewed and non-peer-reviewed academic journal articles, conference papers, theses, book chapters and monographs. Driven by the problems of social inequality caused by restricting access to academic research, the Open Access movement changes the funding system of published literature allowing for more readers and thus increased access to scientific knowledge, as well as addressing the economic challenges and unsustainability of academic publishing. In addition to being free to read (gratis), open access articles may also be free to use (libre) where the copyright is held by the authors and not the publisher. Definition by the Directory of Open Access Journals (DOAJ): "We define these as journals where the copyright holder of a scholarly work grants usage rights to others using an open license (Creative Commons or equivalent) allowing for immediate free access to the work and permitting any user to read, download, copy, distribute, print, search, or link to the full texts of articles, crawl them for indexing, pass them as data to software, or use them for any other lawful purpose." |
| Open Science | OS | Building on the essential principles of academic freedom, research integrity and scientific excellence, open science sets a new paradigm that integrates into the scientific enterprise practices for reproducibility, transparency, sharing and collaboration resulting from the increased opening of scientific contents, tools and processes. Open science is defined as an inclusive construct that combines various movements and practices aiming to make multilingual scientific knowledge openly available, accessible and reusable for everyone, to increase scientific collaborations and sharing of information for the benefits of science and society, and to open the processes of scientific knowledge creation, evaluation and communication to societal actors beyond the traditional scientific community. It comprises all scientific disciplines and aspects of scholarly practices, including basic and applied sciences, natural and social sciences and the humanities, and it builds on the following key pillars: open scientific knowledge, open science infrastructures, science communication, open engagement of societal actors and open dialogue with other knowledge systems. |
| Open chamber | O | The term "open O2k-chamber" refers to a situation in which the liquid phase is allowed to equilibrate with a gas phase, but the stopper is partially inserted using the Stopper-Spacer. |
| Ordinate | y | The ordinate is the vertical axis y of a rectangular two-dimensional graph with the abscissa x as the horizontal axis. Values Y are placed vertically from the origin. |
| Ouabain | Oua | Ouabain (synonym: G-strophantin octahydrate) is a poisonous cardiac glycoside. The classical mechanism of action of ouabain involves its binding to and inhibition of the plasma membrane Na+/K+-ATPase (sodium pump) especially at the higher concentrations. Low (nanomolar and subnanomolar) concentrations of ouabain stimulate the Na-K-ATPase. |
| Outlier-skewness index | OSI, OI | An outlier-skewness index OSI is defined for evaluation of the distribution of data sets with outliers including separate clusters or skewness in relation to a normal distribution with equivalence of the average and median. The OSI is derived from Pearson’s coefficient of skewness 2:
The outlier-skewness index OSI introduces the absolute value of the arithmetic mean, m = ABS(average + median)/2, for normalization:
At the limit of a zero value of m, the OSI equals the Pearson 2 coefficient (without the multiplication factor of 3). At high m with small standard deviation (SD), the OSI is effectively the difference between the average and the median normalized for m, (average-median)/m. |
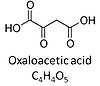
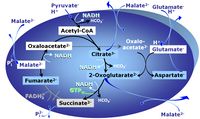
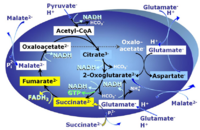
| Oxaloacetate | Oa |
Oxaloacetic acid, C4H4O5, occurs under physiological conditions as the anion oxaloacetate2-, Oa. Oxaloacetate is formed from malate by MDH. Oa reacts with acetyl-CoA through citrate synthase to form citrate, or with glutamate through transaminase to form oxoglutarate and aspartate. Oa transport is restricted across the inner mt-membrane of various tissues. Oa is a potent inhibitor of succinate dehydrogenase. |
| Oxidative phosphorylation | OXPHOS | |
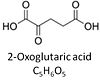
| Oxoglutarate | Og |
2-Oxoglutaric acid or alpha-ketoglutaric acid, C5H6O5, occurs under physiological conditions as the anion 2-Oxoglutarate2-, Og. 2-Oxoglutarate (alpha-ketoglutarate) is formed from isocitrate as a product of isocitrate dehydrogenase (IDH) in the TCA cycle, and is a substrate of oxoglutarate dehydrogenase (OgDH). The 2-oxoglutarate carrier exchanges malate2- for 2-oxoglutarate2- as part of the malate-aspartate shuttle. In the cytosol, oxoglutarate+aspartate are transaminated to form oxaloacetate+glutamate. Cytosolic malate dehydrogenase converts oxaloacetate+NADH to malate. |
| Oxoglutarate dehydrogenase | OgDH | Oxoglutarate dehydrogenase (α-ketoglutarate dehydrogenase) is a highly regulated enzyme of the tricarboxylic acid cycle. It catalyses the conversion of oxoglutarate (alpha-ketoglutarate) to succinyl-CoA, reduces NAD+ to NADH and thus links to Complex I in the Electron transfer-pathway. OgDH is activated by low Ca2+ (<20 µM) but inactivated by high Ca2+ (>100 µM). OgDH is an important source of ROS. |
| Oxycaloric equivalent | DeltakHO2 | The oxycaloric equivalent is the theoretically derived enthalpy change of the oxidative catabolic reactions per amount of oxygen respired, DeltakHO2, ranging from -430 to -480 kJ/mol O2. The oxycaloric equivalent is used in indirect calorimetry to calculate the theoretically expected metabolic heat flux from the respirometrically measured metabolic oxygen flux. Calorimetric/respirometric ratios (CR ratios; heat/oxygen flux ratios) are experimentally determined by calorespirometry. A CR ratio more exothermic than the oxycaloric equivalent of -480 kJ/mol indicates the simultaneous involvement of aerobic and anaerobic mechanisms of energy metabolism. |
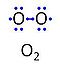
| Oxygen | O2 |
Molecular oxygen, O2 or dioxygen, has two atoms of oxygen, O, which is the chemical element with atomic number 8. The relative molecular mass of O2, Mr,O2, is 32 (or 31.9988). The element O has 8 protons, 8 neutrons and 8 electrons. In the figure, the two electrons in the first electron shell are not shown. Of the six electrons in the outer shell (blue bullets), one electron from each of the two atoms is shared in O2 forming the covalent bond, and one electron in each atom is unpaired. |
| Oxygen flow | IO2 [mol·s-1] or [mol·s-1·x-1] | Respiratory oxygen flow is the oxygen consumption per total system, which is an extensive quantity. Flow is advancement of a transformation in a system per time [mol·s-1], when 'system' is defined as the experimental system (e.g. an open or closed chamber). Flow is distinguished from the size-specific quantity flux obtained by normalization of flow per volume of the experimental system [mol·s-1·m-3]. An experimental object, e.g. a living cell, may be considered as the 'experimental system'. Then oxygen flow per cell has the unit [mol·s-1·x-1], where [x] is the elementary unit for a count. Oxygen flow or respiration per cell [amol·s-1·x-1] = [pmol·s-1·Mx-1] is normalized for the cell count, distinguished from oxygen flux (e.g. per mg protein or wet mass). These are different forms of normalization of rate. |
| Oxygen flux | JO2 | Oxygen flux, JO2, is a specific quantity. Oxygen flux is oxygen flow, IO2 [mol·s-1 per system] (an extensive quantity), divided by system size. Flux may be volume-specific (flow per volume [pmol·s-1·mL-1]), mass-specific (flow per mass [pmol·s-1·mg-1]), or marker-specific (flow per mtEU). Oxygen flux (e.g., per body mass, or per cell volume) is distinguished from oxygen flow (per number of objects, such as cells), IO2 [mol·s-1·x-1]. These are different forms of normalization of rate. |
| Oxygen flux - instrumental background | J°O2 | Instrumental background oxygen flux, J°O2, in a respirometer is due to oxygen consumption by the POS, and oxygen diffusion into or out of the aqueous medium in the O2k-chamber. It is a property of the instrumental system, measured in the range of experimental oxygen levels by a standardized instrumental O2 background test. The oxygen regime from air saturation towards zero oxygen is applied generally in experiments with isolated mitochondria, and living or permeabilized cells. To overcome oxygen diffusion limitation in permeabilized fibers and homogenates, an elevated oxygen regime is applied, requiring instrumental background test in the same range of elevated oxygen. |
| Oxygen pressure | pO2 [kPa] | Oxygen pressure or partial pressure of oxygen [kPa], related to oxygen concentration in solution by the oxygen solubility, SO2 [µM/kPa]. |
| Oxygen sensor test | POS test | The O2 sensor test is an important component of Oroboros Quality Management. The OroboPOS test is described in detail in MiPNet06.03 POS-calibration-SOP, is performed after switching on the Oroboros O2k, and is required as a basis of technical service of the instrument. |
| Oxygen solubility | SO2 [µM/kPa] | The oxygen solubility, SO2 [µM/kPa] = [(µmol·L-1)/kPa], expresses the oxygen concentration in solution in equilibrium with the oxygen pressure in a gas phase, as a function of temperature and composition of the solution. The inverse of oxygen solubility is related to the activity of dissolved oxygen. The oxygen solubility in solution, SO2(aq), depends on temperature and the concentrations of solutes in solution, whereas the dissolved oxygen concentration at equilibrium with air, cO2*(aq), depends on SO2(aq), barometric pressure and temperature. SO2(aq) in pure water is 10.56 µM/kPa at 37 °C and 12.56 µM/kPa at 25 °C. At standard barometric pressure (100 kPa), cO2*(aq) is 207.3 µM at 37 °C (19.6 kPa partial oxygen pressure) or 254.7 µM at 25 °C (20.3 kPa partial oxygen pressure). In MiR05 and serum, the corresponding saturation concentrations are lower due to the oxygen solubility factor: 191 and 184 µM at 37 °C or 234 and 227 µM at 25 °C. |
| Oxygen solubility factor | FM | The oxygen solubility factor of the incubation medium, FM, expresses the effect of the salt concentration on oxygen solubility relative to pure water. In mitochondrial respiration medium MiR05, MiR05-Kit and MiR06, FM is 0.92 (determined at 30 and 37 °C) and in culture media is 0.89 (at 37 °C). FM varies depending on the temperature and composition of the medium. To determine the FM based on the oxygen concentration, specific methods and equipment are needed (see references Rasmussen HN, Rasmussen UF 2003 in MiPNet06.03). For other media, FM may be estimated using Table 4 in MiPNet06.03. For this purpose KCl based media can be described as "seawater" of varying salinity. The original data on sucrose and KCl-media (Reynafarje et al 1985), however, have been critizesed as artefacts and the FM of 0.92 is suggested in the temperature range of 10 °C to 40 °C as for MiR05. |
| P-L control efficiency | jP-L | |
| P-L net OXPHOS capacity | P-L | |
| P/E control ratio | P/E | |
| P/O ratio | P/O ratio | P/O ratio stands for phosphate to atomic oxygen ratio, where P indicates phosphorylation of ADP to ATP (or GDP to GTP). |
| P50 | p50 | p50 is the oxygen partial pressure at which (a) respiratory flux is 50% of maximum oxygen flux, Jmax, at saturating oxygen levels. The oxygen affinity is indirectly proportional to the p50. The p50 depends on metabolic state and rate. (b) p50 is the oxygen partial pressure at which oxygen binding (on myoglobin, haemoglobin) is 50%, or desaturation is 50%. |
| PBMC | PBMC | Peripheral blood mononuclear cells (PBMC) are a fraction of the leucocyte population in the blood composed by cells with round nucleus. PBMC consist of lymphocytes (T, B and NK cells) and monocytes. During extraction, neutrophils and platelets (PLT) can be found in the PBMC fraction, where PLT are considered as a contamination. |
| PGM-pathway control state | PGM | PGM: Pyruvate & Glutamate & Malate. MitoPathway control state: NADH electron transfer-pathway state Pyruvate (P) is oxidatively decarboxylated to acetyl-CoA and CO2, yielding NADH catalyzed by pyruvate dehydrogenase. Malate (M) is oxidized to oxaloacetate by mt-malate dehydrogenase located in the mitochondrial matrix. Condensation of oxaloacate with acetyl-CoA yields citrate (citrate synthase). Glutamate&malate is a substrate combination supporting an N-linked pathway control state, when glutamate is transported into the mt-matrix via the glutamate-aspartate carrier and reacts with oxaloacetate in the transaminase reaction to form aspartate and oxoglutarate. Glutamate as the sole substrate is transported by the electroneutral glutamate-/OH- exchanger, and is oxidized in the mitochondrial matrix by glutamate dehydrogenase to α-ketoglutarate ( 2-oxoglutarate), representing the glutamate-anaplerotic pathway control state. 2-oxoglutarate (α-ketoglutarate) is formed from isocitrate (isocitrate dehydrogenase, from oxaloacetate and glutamate by the transaminase, and from glutamate by the glutamate dehydrogenase. |
| PGMS-pathway control state | PGMS | PGMS: Pyruvate & Glutamate & Malate & Succinate. MitoPathway control state: NS-pathway control state 2-oxoglutarate is produced through the citric acid cycle from citrate by isocitrate dehydrogenase, from oxaloacetate and glutamate by the transaminase, and from glutamate by the glutamate dehydrogenase. If the 2-oxoglutarate carrier does not outcompete these sources of 2-oxoglutarate, then the TCA cycle operates in full circle with external pyruvate&malate&glutamate&succinate |
| PGMSGp pathway control state | PGMSGp | PGMSGp: Pyruvate & Glutamate & Malate & Succinate & Glycerophosphate. MitoPathway control state: NSGp SUIT protocol: SUIT-038 This substrate combination supports convergent electron flow to the Q-junction. |
| PH | pH | The pH value or pH is the negative of the base 10 logarithm of the activity of protons (hydrogen ions, H+). A pH electrode reports the pH and is sensitive to the activity of H+. In dilute solutions, the hydrogen ion activity is approximately equal to the hydrogen ion concentration. The symbol pH stems from the term potentia hydrogenii. |
| PM-pathway control state | PM | MitoPathway control state: NADH Electron transfer-pathway state
|
| PMS-pathway control state | PMS | PMS: Pyruvate & Malate & Succinate. MitoPathway control: CI&II Pyruvate (P) is oxidatively decarboxylated to acetyl-CoA and CO2, yielding NADH catalyzed by pyruvate dehydrogenase. Malate (M) is oxidized to oxaloacetate by mt-malate dehydrogenase located in the mitochondrial matrix. Condensation of oxaloacate with acetyl-CoA yields citrate (citrate synthase). This documents an additive effect of convergent CI&II electron flow to the Q-junction, with consistent results obtained with permeabilized muscle fibres and isolated mitochondria (Gnaiger 2009). |
| POS calibration - static | F5 | Two-point calibration of the polarographic oxygen sensor, comprising Air calibration and Zero calibration. See also POS calibration - dynamic. |
| POS-Membranes | FEP-membranes | POS-Membranes, FEP 25 µm; 40/Pck. |
| PalM | PalM | PalM: Palmitoylcarnitine & Malate. MitoPathway control state: Fatty acid oxidation pathway control state SUIT protocols: SUIT-019 |
| PalOctM | PalOctM | PalOctM: Palmitoylcarnitine & Octanoylcarnitine & Malate. MitoPathway control state: Fatty acid oxidation pathway control state SUIT protocols: SUIT-019 |
| PalOctPGM | PalOctPGM | PalOctPGM: Palmitoylcarnitine & Octanoylcarnitine & Pyruvate & Glutamate & Malate. MitoPathway control state: FN SUIT protocols: SUIT-019 |
| PalOctPGMS | PalOctPGMS | PalOctPGMS: Palmitoylcarnitine & Octanoylcarnitine & Pyruvate & Glutamate & Malate & Succinate. MitoPathway control state: FNS SUIT protocols: SUIT-019 |
| PalOctPM | PalOctPM | PalOctPM: Palmitoylcarnitine & Octanoylcarnitine & Pyruvate & Malate. MitoPathway control state: FN SUIT protocols: SUIT-019 |
| PalPGMSGp pathway control state | PalPGMSGp | PalPGMSGp: Palmitoylcarnitine & Pyruvate & Glutamate & Malate & Succinate & Glycerophosphate. MitoPathway control state: FNSGp SUIT protocol: SUIT-026 This substrate combination supports convergent electron flow to the Q-junction. |
| Palmitate | Paa | Palmitate is a term for the salts and esters of palmitic acid (CH3(CH2)14COOH). Palmitic acid is the first fatty acid produced during fatty acid synthesis and the precursor to longer fatty acids. Palmitate negatively feeds back on acetyl-CoA carboxylase (ACC), which is responsible for converting acetyl-CoA to malonyl-CoA, which in turn is used to add to the growing acyl chain, thus preventing further palmitate generation. In order to dissolve the water-insoluble sodium palmitate, BSA is needed to form the water-soluble compound called palmitate:BSA. |
| Palmitoyl-CoA | Pa-CoA | Palmitoyl-CoA is a coenzyme A derivative of palmitate formed by acyl-CoA synthase. In contrast to medium- and short-chain acyl-CoA, palmitoyl-CoA cannot freely diffuse into the mitochondrial matrix. Formation of palmitoylcarnitine by CPTI is necessary prior to transfer into mitochondria for further fatty acid oxidation (β-oxidation). To study Fatty acid oxidation using Palmitoyl-CoA, Carnitine and low amount of malate is needed on mitochondrial preparations. |
| Palmitoylcarnitine | Pal | Palmitoylcarnitine is an ester derivative of carnitine (long-chain acylcarnitine) involved in the metabolism of fatty acids. Within the cell, palmitoylcarnitine is transported into the mitochondria to deliver palmitate for fatty acid oxidation and energy production. |
| Partial oxygen pressure | pO2 [kPa] | The partial oxygen pressure pO2 [kPa] is the contribution of the O2 gas pressure to the total gas pressure. According to the gas law, the partial oxygen pressure is pO2(g) = nO2(g)·V·RT, where the concentration is cO2(g) = nO2(g)·V-1 [mol·m-3], R is the gas constant, and T is the absolute temperature, and RT is expressed in units of chemical force [J·mol-1]. In aqueous solutions at equilibrium with a gas phase, the partial O2 pressures are equal in the aqueous phase (aq) and gas phase (g), pO2(aq) = pO2(g) at total pressures where the partial pressure equals the fugacity. The O2 concentration in the aqueous phase, however, is much lower than in the gas phase, due to the low oxygen solubility in water. The activity of dissolved O2 is expressed by the pO2, where the solubility can be seen as an activity coefficient. |
| Particle charge | QNX, QNX | The particle charge QNX (QNX) or charge per elementary entity is the charge QelX [C] carried by ions of type X divided by the count NX [x]. The particle charge per proton is the elementary charge or proton charge e. |
| Pascal | Pa | The pascal [Pa] is the SI unit for pressure. [Pa] = [J·m-3] = [N·m-2] = [m-1·kg·s-2]. The standard pressure is 100 kPa = 1 bar (105 Pa; 1 kPa = 1000 Pa). Prior to 1982 the standard pressure has been defined as 101.325 kPa or 1 standard atmosphere (1 atm = 760 mmHg). |
| Pathway control efficiency | jZ-Y | Pathway control efficiencies are flux control efficiencies, expressing the relative change of flux in response to a transition between two electron-transfer-pathway states due to a change of (1) substrate availability or (2) inhibition of enzyme steps in the pathway, in a defined coupling-control state. |
| Pathway control ratio | FCR | Substrate control ratios are flux control ratios FCR, at a constant mitochondrial coupling-control state. Whereas there are only three well-defined coupling-control states of mitochondrial respiration, L, P, E (LEAK respiration, OXPHOS, Electron transfer pathway), numerous Electron-transfer-pathway states are possible. Careful selection of the reference state, Jref, is required, for which some guidelines may be provided without the possibility to formulate general rules. FCR are best defined by taking Jref as the maximum flux (e.g. NSE), such that flux in various other respiratory states, Ji, is smaller or equal to Jref. However, this is not generally possible with FCR. For instance, the N/S pathway control ratio (at constant coupling-control state) may be larger or smaller than 1.0, depending on the mitochondrial source and various mitochondrial injuries. The S-pathway control state may be selected preferentially as Jref, if mitochondria with variable N-linked injuries are studied. In contrast, the reference state, Z, is strictly defined for flux control efficiency. |
| Perfluorooctanoic acid | PFOA | Perfluorooctanoic acid (PFOA) is a metabolically inert perfluorinated fatty acid which activates UCP1 in brown-fat mitochondria. UCP1-dependent respiration can be stimulated with 600 μM PFOA after inhibition of the phosphorylation system. |
| Permeability transition pore | PTP | The (mitochondrial, mt) permeability transition pore (PTP) is an unspecific pore presumed to involve components of both the inner and outer mt membrane which upon opening induces a massive increase of the inner mt membrane permeability for solutes up to 1.5 kDa. It is crucially involved in cell death induction in response to, among other stimuli, radical stress and/or calcium overload and may cause necrosis or apoptosis. It plays an important role in neurodegenerative diseases, cardiac ischemia-reperfusion injury and possibly various other diseases. Previously considered essential molecular constituents such as the voltage-dependent anion channel (VDAC), the adenine nucleotide translocator (ANT) and cyclophilin D (CypD) have all been shown to be important regulators of mtPTP opening, but the molecular entities actually forming the pore are still unknown at present. The opening of the pore can be prevented using cyclosporin A, a compound that binds cyclophilin D avoiding the formation of the pore. In respirometry, mtPTP opening may be observed as a sudden decrease of respiration of isolated mitochondria (Hansson 2010 J Biol Chem). |
| Permeabilized cells | pce | Permeabilized cells (pce) are mitochondrial preparations obtained by selectively permeabilizing the plasma membrane (e.g., with digitonin), for the exchange of soluble molecules between the cytosolic phase and external medium, without damaging the mt-membranes. Permeabilized cells (pce) are, therefore, not any longer viable or living cells (ce), since the intactness of cells implies the intactness of the plasma membrane. Any typical quantiative cell viability test (trypan blue etc) evaluating the intactness of the plasma membrane, yields a 100% negative result on fully permeabilized cells. For permeabilizing the cell plasma membranes chemically with digitonin, without damaging the mt-membranes, the optimum concentration of digitonin must be previously determinated. The protocol SUIT-010 is designed for the evaluation of optimum digitonin concentration for permeabilizing cells, a requirement to account for differences between cell types, the concentration of cells, and variability between batches of the natural product digitonin. |
| Permeabilized muscle fibers | pfi | Permeabilized muscle fibers (pfi) are used as a mitochondrial preparation in respirometry to access mitochondrial function comparable to isolated mitochondria (imt). pfi are obtained by selectively permeabilizing the plasma membrane mechanically and chemically (saponin), for the exchange of soluble molecules between the cytosolic phase and external medium, without damaging the mt-membranes.
|
| Permeabilized tissue | pti | Permeabilized tissue (pti, see also permeabilized muscle fibers, pfi) are mitochondrial preparations obtained by selectively permeabilizing the plasma membrane mechanically or chemically (e.g., with saponin), for the exchange of soluble molecules between the cytosolic phase and external medium, without damaging the mt-membranes. |
| Permeabilized tissue or cells | pti, pce | Permeabilized tissue (pti, see also permeabilized muscle fibers, pfi) or cells (pce) are mitochondrial preparations obtained by selectively permeabilizing the plasma membrane mechanically or chemically, for the exchange of soluble molecules between the cytosolic phase and external medium, without damaging the mt-membranes. Permeabilized cells (pce) are, therefore, not any longer viable or living cells (ce), since the intactness of cells implies the intactness of the plasma membrane. Any typical quantiative cell viability test (trypan blue etc) evaluating the intactness of the plasma membrane, yields a 100% negative result on fully permeabilized cells. |
| Peroxisome proliferator-activated receptor gamma coactivator 1-alpha | PGC-1α | Peroxisome proliferator-activated receptor-γ (PPAR-γ) coactivator-1α (PGC-1α) is a protein which functions as an inducible transcriptional coactivator, a coregulator of transcription factors, particularly NRF-1 and TFAM. PGC-1α was first described in 1998 (Puigserver 1998 Cell). PGC-1α drives the formation of slow-twich muscle fibres (Lin 2002 Nature) and is increased upon endurance training (Norrbom 2004 J Appl Physiol). PGC-1α expression is inhibited by the proinflammatory cytokine tumor necrosis factor α (TNF-α) and high levels of leptin. Upregulation of PGC-1α expression is induced by increased eNOS activity -> NO -> guanylate cyclase -> cGMP (Nisoli 2007 Circ Res). AMP-activated protein kinase (AMPK) increases PGC-1α expression through SIRT1 (Canto 2009 Nature). |
| Phosphate | Pi | See: Inorganic phosphate |
| Phosphate carrier | PiC | The phosphate carrier (PiC) is a proton/phosphate symporter which transports negatively charged inorganic phosphate across the inner mt-membrane. The transport can be described either as symport of H+ with Pi, or antiport of hydroxide anion against Pi. The phosphate carrier is a component of the phosphorylation system. |
| Phosphocreatine | PCr | Phosphocreatine is a high energy compound in the skeletal muscle of vertebrates and is present in 4 to 5 times the concentration of ATP. |
| Phosphoenolpyruvate carboxykinase | PEPCK | Phosphoenolpyruvate carboxykinase (PEPCK) catalyzes the anabolic reaction of oxaloacetate (Oxa) to phosphoenolpyruvate at the expense of GTP. PEPCK is a cytoplasmatic enzyme involved in gluconeogenesis in mouse and rat liver, but 'is found in the mitochondria in the rabbit and chicken, and in both cytoplasm and mitochondria in the guinea pig' (Lehninger 1970). In many anoxia-resistant animals, PEPCK plays an important catabolic role under severe hypoxia and anoxia at the PEPCK branchpoint (Hochachka, Somero 2002), feeding malate into the reversed TCA cycle: malate is dismutated to pyruvate catalyzed by malic enzyme in the oxidative direction, and to fumarate in the reductive direction, leading to formation of succinate and ATP under anoxia (Gnaiger 1977). |
| Phosphorylation pathway | DT |
The phosphorylation pathway (phosphorylation system) is the functional unit utilizing the protonmotive force to phosphorylate ADP (D) to ATP (T), and may be defined more specifically as the P»-system. The P»-system consists of adenine nucleotide translocase, phosphate carrier, and ATP synthase. Mitochondrial adenylate kinase, mt-creatine kinase and mt-hexokinase constitute extended components of the P»-system, controlling local AMP and ADP concentrations and forming metabolic channels. Since substrate-level phosphorylation is involved in the TCA-cycle, the P»-system includes succinyl-CoA ligase (GDP to GTP or ADP to ATP). |
| PhotoBiology | PB | PhotoBiology is the science of the effect of light on biological processes. This includes photosynthesis, photochemistry, photophysics, photomorphogenesis, vision, bioluminescence, circadian rhythms and photodynamic therapy. Phototoxicity results from non-ionizing radiation (i.e. ultraviolet, visible and infrared radiation). Non-ionizing radiation is any type of electromagnetic radiation that does not carry enough energy per quantum (photon energy below 10 eV) to completely remove an electron from an atom or molecule. When photons interact with molecules, the molecules can absorb the photon energy and become excited, reacting with surrounding molecules and stimulating "photochemical" and "photophysical" changes. Respiration may be affected by light during photosynthesis or in dark respiration, with the transient response of light-enhanced dark respiration. |
| Photodecomposition | PD | Photodecomposition or photodegradation is the process of decay of organic material induced by increasing light intensity. Under aerobic conditions, the enhancement of photodecomposition by light intensity can be quantified by oxygen consumption in a controlled light regime. |
| Photosynthesis | PS | Photosynthesis is the process that converts light energy into chemical energy which is subsequently transformed to the physiological energy demand. Photosynthesis has a light-dependent and light-independent (dark) phase. In plants, algae, and cynobacteria, light energy is absorbed during the light phase by the pigment chlorophyll and used to split water and generate adenosine triphosphate (ATP) and reducing power - nicotinamide adenine dinucleotide phosphate (NADPH), with the net production of O2 as a waste product. During the dark phase ATP and NADPH are used to synthesize carbohydrates from CO2 through the metabolic pathway called Calvin-Benson cycle. Oxygenic photosynthesis is responsible for producing and maintaining the oxygen concentration of the Earth’s atmosphere. In bacteria such as cyanobacteria, photosynthesis involves the plasma membrane and the cytoplasm. In eukaryotic cells (plants and algae), photosynthesis takes place in the chloroplasts. |
| Plan S | S | Plan S is an initiative for Open Access publishing that was launched in September 2018. The plan is supported by cOAlition S, an international consortium of research funding and performing organisations. Plan S requires that, from 2021, scientific publications that result from research funded by public grants must be published in compliant Open Access journals or platforms. According to Science Europe, "Plan S requires that recipients of research funding from cOAlition S organisations make the resulting publications available immediately (without embargoes) and under open licences, either in quality Open Access platforms or journals or through immediate deposit in open repositories that fulfil the necessary conditions." |
| Platelet | PLT | Platelets or thrombocytes (PLT) are cell fragments derived from megakaryocytes with hemostatic function in the blood stream. PLT are anucleated but contain functioning mitochondria that play a critical role in PLT activation. |
| Platelet-rich plasma | PRP | Platelet-rich plasma (PRP) is obtained as the upper layer at low-speed centrifugation (around 150-200 g), when white and red blood cells sediment and thus get separated from plasma containing the platelets. For further details see blood cell preparation. |
| Plot - DatLab | Ctrl+F6 | A plot in DatLab represents a specific channel in the graph. To change the Layout for DatLab graphs go to [Graph]/Select plots to open the Graph layout window. |
| Polarization voltage | U | A polarization voltage of 600 mV to 800 mV is applied between anode and cathode of the polarographic oxygen sensor, resulting in a current when oxygen is consumed. The current is converted by the electronics to a voltage (raw signal) which must not be confused with the polarization voltage. |
| Polarographic oxygen sensor | POS | Polarographic oxygen sensors (POS) are operated with a polarization voltage between the cathode and anode, connected by an electrolyte. Cathode, anode and electrolyte are separated from the analyte by an oxygen-permeable membrane. Oxygen is reduced at the cathode such that the local oxygen concentration is maintained at zero, and diffuses along the concentration gradient from the stirred medium to the cathode, resulting in a linear calibration between oxygen partial pressure and electric current [Amp] (amperometric mode of operation). The OroboPOS is the POS applied in the Oroboros O2k. |
| Polyether ether ketone | PEEK | Polyether ether ketone (PEEK) is a semicrystalline organic polymer thermoplastic, which is chemically very resistant, with excellent mechanical properties. PEEK is compatible with ultra-high vacuum applications, and its resistance against oxygen diffusion make it an ideal material for high-resolution respirometry (POS insulation; coating of stirrer bars; stoppers for closing the O2k-Chamber). |
| Polyvinylidene fluoride | PVDF | Polyvinylidene fluoride (PVDF) is a pure thermoplastic fluoropolymer, which is chemically very resistant, with excellent mechanical properties. It is used generally in applications requiring the highest purity, strength, and resistance to solvents, acids, bases and heat (Wikipedia). PVDF is resistant against oxygen diffusion which makes it an ideal material for high-resolution respirometry (coating of stirrer bars; stoppers for closing the O2k-Chamber). |
| Power | P [W] | Power P [W = J·s-1] is exergy per time, or force times flow, which cannot be created internally yet is not conserved but is dissipated (P < 0) in irreversible energy transformations at constant temperature and (barometric) pressure, T,p. Metabolic power and heat flux of irreversible processes are distinguished as the time rate of Gibbs energy and enthalpy changes, respectively. |
| Pressure | p, P, Π [Pa] | Pressure is a fundamental quantity expressing energy per volume. The SI unit of pressure is generally pascal [Pa] = [J·m-3]. The term 'stress' (mechanical stress) is used as a synonym for pressure (SI). Pressure is known in physics as mechanical pressure, which is force per area, p = F·A-1 [Pa] = [N·m-2]. In physical chemistry, gas pressure is defined as p = n·V-1·RT, where the concentration is c = n·V-1 [mol·m-3], R is the gas constant, and T is the absolute temperature, and RT is expressed in units of chemical force [J·mol-1]. van't Hoff's osmotic pressure assumes the same form applied to dissolved substances diffusing across a semipermeable membrane, but concentrations should be replaced by activities. The activity of dissolved gases is expressed by the partial pressure, where the solubility can be seen as an activity coefficient. Pressure appears explicitely or implicitely in all chapters of physics and physical chemistry. In contrast to the universal counterparts energy and force, however, the general connections between various isomorphic expressions of pressure remain poorly understood: Pressure is the concentration of the force at the point of action. More generally, pressure is the force times concentration at the interphase of interaction. |
| Proficiency test | PT | Proficiency testing PT is an evaluation of participant performance against pre-established criteria by means of interlaboratory comparisons. Some PT providers in the medical area use the term “External Quality Assessment (EQA)” for their proficiency testing schemes, or for their broader programmes, or both. Internal PT strategies may be implemented into laboratory science as practical steps towards PT to achieve reproducibility. |
| Proline | Pro |
Proline (Pro), C5H9NO2, is an amino acid which occurs under physiological conditions mainly in the nonpolar form, with pKa1 = 1.99 pKa2 = 10.96. Proline is an anaplerotic substrate that supports both the proline pathway control state and the glutamate-anaplerotic pathway control state. Proline is used as a single substrate or in combination with carbohydrate-derived metabolites in mitochondria particularly of flight muscle of many (but not all) insects. Proline is oxidized to delta-1-pyrroline-5-carboxylate by the mtIM L-proline:quinone oxidoreductase (proline dehydrogenase, ProDH), with reduction of FAD to FADH2 and direct entry into the Q-junction. delta-1-pyrroline-5-carboxylate is converted to glutamate by 1-pyrroline-5-carboxylate dehydrogenase. |
| Proline dehydrogenase | ProDH | Proline dehydrogenase (ProDH), L-proline:quinone oxidoreductase, is located on the inner side of the mtIM, oxidizing proline to delta-1-pyrroline-5-carboxylate, with reduction of FAD to FADH2 and direct entry into the Q-junction, exerting an additive effect of convergent pathways. ProDH is widely distributed in a variety of organisms, is a source of ROS, and may play a role in carcinogenesis. |
| Proton | p+, p | The terms proton p and hydrogen ion H+ are used synonymously in chemistry. In particle physics, a proton is a subatomic particle with a positive electric charge. Protons and neutrons are collectively referred to as nucleons. The proton is a bare charge with only about 1/64 000 of the radius of a hydrogen atom, and so the free proton is extremely reactive chemically. Therefore, the free proton has an extremely short lifetime in aqueous solutions where it forms the hydronium ion, H3O+, which in turn is further solvated by water molecules in clusters such as H5O2+ and H9O4+. |
| Protonmotive force | pmF, ∆mFH+, Δp [J·MU-1] | The protonmotive force ∆mFH+ is known as Δp in Peter Mitchell’s chemiosmotic theory [1], which establishes the link between electric and chemical components of energy transformation and coupling in oxidative phosphorylation. The unifying concept of the pmF ranks among the most fundamental theories in biology. As such, it provides the framework for developing a consistent theory and nomenclature for mitochondrial physiology and bioenergetics. The protonmotive force is not a vector force as defined in physics. This conflict is resolved by the generalized formulation of isomorphic, compartmental forces, ∆trF, in energy (exergy) transformations [2]. Protonmotive means that there is a potential for the movement of protons, and force is a measure of the potential for motion. The pmF is generated in oxidative phosphorylation by oxidation of reduced fuel substrates and reduction of O2 to H2O, driving the coupled proton translocation from the mt-matrix space across the mitochondrial inner membrane (mtIM) through the proton pumps of the electron transfer pathway (ETS), which are known as respiratory Complexes CI, CIII and CIV. ∆mFH+ consists of two partial isomorphic forces: (1) The chemical part, ∆dFH+, relates to the diffusion (d) of uncharged particles and contains the chemical potential difference§ in H+, ∆µH+, which is proportional to the pH difference, ∆pH. (2) The electric part, ∆elFp+ (corresponding numerically to ∆Ψ)§, is the electric potential difference§, which is not specific for H+ and can, therefore, be measured by the distribution of any permeable cation equilibrating between the negative (matrix) and positive (external) compartment. Motion is relative and not absolute (Principle of Galilean Relativity); likewise there is no absolute potential, but isomorphic forces are stoichiometric potential differences§. The total motive force (motive = electric + chemical) is distinguished from the partial components by subscript ‘m’, ∆mFH+. Reading this symbol by starting with the proton, it can be seen as pmF, or the subscript m (motive) can be remembered by the name of Mitchell, ∆mFH+ = ∆dFH+ + ∆elFp+ With classical symbols, this equation contains the Faraday constant, F, multiplied implicitly by the charge number of the proton (zH+ = 1), and has the form [1] ∆p = ∆µH+∙F-1 + ∆Ψ A partial electric force of 0.2 V in the electrical format, ∆elFeH+a, is 19 kJ∙mol-1 H+a in the molar format, ∆elFnp+a. For 1 unit of ∆pH, the partial chemical force changes by -5.9 kJ∙mol-1 in the molar format, ∆dFnH+a, and by 0.06 V in the electrical format, ∆dFeH+a. Considering a driving force of -470 kJ∙mol-1 O2 for oxidation, the thermodynamic limit of the H+a/O2 ratio is reached at a value of 470/19 = 24, compared to the mechanistic stoichiometry of 20 for the N-pathway with three coupling sites. |
| Protonmotive pressure | pmP, ∆mΠH+ [kPa] | The protonmotive pressure, ∆mΠH+ or pmP [kPa], is an extension of Peter Mitchell’s concept of the protonmotive force pmF, based on Fick’s law of diffusion and Einstein’s diffusion equation, accounting for osmotic pressure (corresponding to the diffusion term in the pmF) and electric pressure (the electric term or membrane potential in the pmF). The linearity of the generalized flow-pressure relationship explains the non-ohmic flow-force dependence in the proton leak rate as a function of membrane potential. The total motive pressure (motive = electric + chemical) is distinguished from the partial components by subscript ‘m’, ∆mΠH+, ∆mΠH+ = ∆dΠH+ + ∆elΠp+ |
| Publication efficiency | Fr,a/p | Publication efficiency is the fraction Fr,a/p of reproducible publications Nr which are among the number Na of publications that receive attention and meaningful interpretation, per total count Np of all published communications. Publication efficiency Fr,a/p = Fr/p·Fa/p is low due to (1) the reproducibility crisis expressed as low reproducibility efficiency Fr/p = Nr/Np, and (2) the inflation crisis expressed as low attention efficiency Fa/p = Na/Np. Estimates of these partial efficiencies vary from field to field. With Fr/p=0.15 and Fa/p=0.05, the current publication efficiency is as low as 0.0075, or only 0.75 % of all presently published communictions are reproducible and receive attention and meaningful interpretation. Reduction of the number of irreproducible zero-value publications is the most effective measure to reduce the paper mass excess (PME) in the reproducibility-inflation (R&I)-crisis. Several regulatory mechanisms for improvement are practically ignored although theoretically available. |
| Publicly deposited protocols | PDP | Researchers need to be introduced into adhering to publicly deposited protocols. Prespecified and time-stamped protocols that are publicly deposited may help to save Millions of Euros that may otherwise be wasted on research that is lacking coherent standards. |
| Pyruvate | P |
Pyruvic acid, C3H4O3, is an alpha-keto monocarboxylic acid which occurs under physiological conditions mainly as the anion pyruvate-, P, with pKa = 2.5. Pyruvate is formed in glycolysis from phosphoenolpyruvate. In the cytosol, pyruvate is a substrate of lactate dehydrogenase. Pyruvate enters the mitochondrial matrix via a specific low Km' H+/monocarboxylate cotransporter known as the pyruvate carrier. Similarly, the plasma membrane of many cell types has H+/monocarboxylate cotransporter activity and pyruvate can thus be added as a substrate to living cells. In the mt-matrix the oxidative decarboxylation of pyruvate is catalyzed by pyruvate dehydrogenase and yields acetyl-CoA. Pyruvate competitively reverses the inhibition of cytochrome c oxidase by cyanide. Pyruvate is an antioxidant reacting with hydrogen peroxide. |
| Pyruvate carboxylase | PC | Pyruvate carboxylase synthesizes oxaloacetate from pyruvate and CO2 as an anaplerotic reaction in the mitochondrial matrix of the liver and kidney of higher animals, representing an alternative to the malic enzyme pathway to oxaloacetate or the phosphoenolpyruvate carboxykinase reaction (compare glyoxylate cycle in plants and microorganisms). Carboxylation of pyruvate to oxaloacetate requires Mg-ATP. Acetyl CoA is a strong positive modulator. PC can form pyruvate from oxaloacetate to remove an excess of oxaloacetate which inhibits succinate dehydrogenase. |
| Pyruvate dehydrogenase | PDH | Pyruvate dehydrogenase is the first component enzyme of the pyruvate dehydrogenase complex, which catalyzes oxidative decarboxylation of pyruvate in the mt-matrix, and yields acetyl-CoA. PDH is known as the mitochondrial gatekeeper in the core energy pathway of electron flow into the tricarboxylic acid cycle. |
| Pyruvate dehydrogenase complex | PDHC | Oxidative decarboxylation of pyruvate is catalyzed by the pyruvate dehydrogenase complex in the mt-matrix, and yields acetyl-CoA. |
| P»-system | P»system | The ADP-ATP phosphorylation system or P»-system. See Phosphorylation system. |
| Q | Q | Multiple meanings of Q
|
| Q-Module | Q-Module | The Q-Module, developed for measuring the Q redox state and cyclic voltammetry, is supported by the NextGen-O2k and consists of the Q-Sensor, integrated electronic components in the O2k, and the DatLab software. |
| Q-cycle | Q | Q-cycle refers to the sequential oxidation and reduction of the electron carrier Coenzyme Q (CoQ or ubiquinone) in mitochondria or plastoquinones in the photosynthetic system. Originally, the concept of the Q-cycle was proposed by Peter D Mitchell. Following several modifications, the Q-cycle is established, describing how CIII translocates hydrogen ions against the protonmotive force. The reduced CoQ (ubiquinol QH2) binds to the Qo site of CIII, while the oxidized CoQ (ubiquinone Q) to the Qi site of CIII. First, QH2 reduces the iron-sulfur protein and feeds cytochrome c1 with one electron. The other electron is transferred to the bL heme and reduces the bH heme, which transfers the electron to ubiquinone at the Qi-site which is reduced to a semiquinone. A second QH2 is required to fully reduce semiquinone to ubiquinol. At the end of the Q-cycle, four protons leave the mt-matrix and enter the intermembrane space, and the reduced cytochrome c transfers electrons to CIV. The ubiquinol generated at the Qi-site can be reused by binding to the Qo-site of CIII. |