Cuperus 2010 Cell Mol Life Sci
| Cuperus R, Leen R, Tytgat GA, Caron HN, van Kuilenburg AB (2010) Fenretinide induces mitochondrial ROS and inhibits the mitochondrial respiratory chain in neuroblastoma. Cell Mol Life Sci 67:807-16. https://doi.org/10.1007/s00018-009-0212-2 |
Cuperus R, Leen R, Tytgat GA, Caron HN, van Kuilenburg AB (2010) Cell Mol Life Sci
Abstract: Fenretinide induces apoptosis in neuroblastoma by induction of reactive oxygen species (ROS). In this study, we investigated the role of mitochondria in fenretinide-induced cytotoxicity and ROS production in six neuroblastoma cell lines. ROS induction by fenretinide was of mitochondrial origin, demonstrated by detection of superoxide with MitoSOX, the scavenging effect of the mitochondrial antioxidant MitoQ and reduced ROS production in cells without a functional mitochondrial respiratory chain (Rho zero cells). In digitonin-permeabilized cells, a fenretinide concentration-dependent decrease in ATP synthesis and substrate oxidation was observed, reflecting inhibition of the mitochondrial respiratory chain. However, inhibition of the mitochondrial respiratory chain was not required for ROS production. Co-incubation of fenretinide with inhibitors of different complexes of the respiratory chain suggested that fenretinide-induced ROS production occurred via complex II. The cytotoxicity of fenretinide was exerted through the generation of mitochondrial ROS and, at higher concentrations, also through inhibition of the mitochondrial respiratory chain.
• Bioblast editor: Gnaiger E
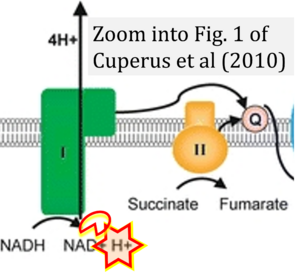
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.