Brischigliaro 2021 Biochim Biophys Acta Bioenerg
| Brischigliaro M, Zeviani M (2021) Cytochrome c oxidase deficiency. Biochim Biophys Acta Bioenerg 1862:148335. doi: 10.1016/j.bbabio.2020.148335 |
Brischigliaro M, Zeviani M (2021) Biochim Biophys Acta Bioenerg
Abstract: Cytochrome c oxidase (COX) deficiency is characterized by a high degree of genetic and phenotypic heterogeneity, partly reflecting the extreme structural complexity, multiple post-translational modification, variable, tissue-specific composition, and the high number of and intricate connections among the assembly factors of this enzyme. In fact, decreased COX specific activity can manifest with different degrees of severity, affect the whole organism or specific tissues, and develop a wide spectrum of disease natural history, including disease onsets ranging from birth to late adulthood. More than 30 genes have been linked to COX deficiency, but the list is still incomplete and in fact constantly updated. We here discuss the current knowledge about COX in health and disease, focusing on genetic aetiology and link to clinical manifestations. In addition, information concerning either fundamental biological features of the enzymes or biochemical signatures of its defects have been provided by experimental in vivo models, including yeast, fly, mouse and fish, which expanded our knowledge on the functional features and the phenotypical consequences of different forms of COX deficiency.
• Bioblast editor: Gnaiger E
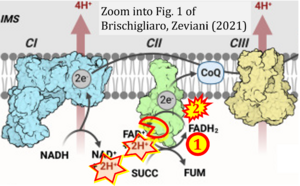
Correction: FAD but not FAD+
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.
Labels:
Enzyme: Complex II;succinate dehydrogenase, Complex IV;cytochrome c oxidase